Human Hibernation
- Posted 02.26.11
- NOVA scienceNOW
They've been called medical miracles: People submerged in icy water, or buried in snow, with no breath or heartbeat. They seem dead, yet a fortunate few are revived—thanks to the cold. Now, across the country, ER doctors are intentionally chilling their patients into hypothermia; meanwhile, scientists are hoping that a cocktail of drugs inspired by hibernating animals could one day perform the same "miracles" on demand.
Transcript
Can We Live Forever?
PBS Airdate: January 26, 2011
NEIL DEGRASSE TYSON (Astrophysicist, American Museum of Natural History): Hi, I'm Neil deGrasse Tyson, your host of NOVA scienceNOW, where this season we're asking six big questions. On this episode: Can We Live Forever?
Some folks seem to be built to last. This guy is 91!
CHUCK YOGI (Honolulu Heart Program Participant): I'm 96.
WOMAN (Jewish Centenarian): Ninety-seven.
SAMUEL HARANO (Honolulu Heart Program Participant): Ninety-eight.
NEIL DEGRASSE TYSON: These people live long and healthy lives. So what's their secret? And where can I get some?
The answer may lie in these guys.
CYNTHIA KENYON (University of California, San Francisco): They're like 90-year-old people who look 45.
NEIL DEGRASSE TYSON: And what if you could replace your broken down human organs as easily as you replace the muffler on your car? Researchers insist that day is coming, and sooner than you think.
DORIS TAYLOR (University of Minnesota): I absolutely see a day where there will be jars of kidneys, jars of livers and jars of lungs, whatever it is you need.
NEIL DEGRASSE TYSON: They're growing body parts in the lab.
So if you can make a working living lung, then it seems to me...
HARALD OTT (Massachusetts General Hospital): ...build, literally, any organ.
NEIL DEGRASSE TYSON: Could we cheat death if we were guaranteed replacement parts?
DORIS TAYLOR: It really makes you go, "What is life?" the first time you see something beat that was dead. It's one of those "yes" moments in life.
NEIL DEGRASSE TYSON: Also...
JASON LEIGH (University of Illinois, Chicago): Can I live forever?
NEIL DEGRASSE TYSON: Just in case our human bodies can't live forever, this computer scientist is trying to design virtual replicas, avatars, that will.
JASON LEIGH: An avatar is an instance of yourself that's digital, that will never die.
NEIL DEGRASSE TYSON: Inspired by Star Trek and Superman.
MARLON BRANDO: (As Jor-El, Superman/Film Clip):You do not remember me. I am your father.
NEIL DEGRASSE TYSON: He thinks we can build digital copies of real people that will carry our thoughts, memories and wisdom into the future, for all posterity.
JASON LEIGH'S AVATAR: You mean me?
NEIL DEGRASSE TYSON: All that and more, on this episode of NOVA ScienceNow.
You know, we take it for granted that nothing lasts forever. And that's true of life itself. Every living thing will eventually break down and die. But does it have to be that way? Can we live forever?
We begin our show with a man who seems to have done the impossible. He's completely stopped the natural decay and death that all of us expect; not for himself but for his car.
IRVIN GORDON (Car Owner): My name is Irvin Gordon. My car now has 2,741,000 miles on it.
NEIL DEGRASSE TYSON: You heard him: more than two-point-seven million miles. The Guinness Book of Records says it's the highest mileage automobile in the world.
IRV GORDON: Every time the car goes go out, I break my own record and make it harder for anybody else to catch up.
NEIL DEGRASSE TYSON: And they'd have over four decades of catching up to do. He drove his new Volvo off the lot 44 years ago, and he and his car have been going strong ever since.
IRV GORDON: You don't have to be the fastest to drive a million miles, you got to just hang in there the longest.
NEIL DEGRASSE TYSON: So this is your baby, huh?
IRV GORDON: This is my baby. Don't touch that car.
Watch your knees.
NEIL DEGRASSE TYSON: It's hard to understand how a car can last so long, look so good and ride so well after all those miles. Irv's car has the same mileage as all of the Apollo moon landings combined! How many places on Earth and things to do take you 3,000,000 miles to get there?
IRV GORDON: Commuting 125 miles a day, to and from work.
NEIL DEGRASSE TYSON: One-hundred-twenty-five miles round-trip?
IRV GORDON: Thirty-five years.
NEIL DEGRASSE TYSON: Are you retired now?
IRV GORDON: I'm retired.
NEIL DEGRASSE TYSON: So, retired from what?
IRV GORDON: I'm retired 12 years ago.
NEIL DEGRASSE TYSON: From what?
IRV GORDON: I was a science teacher.
NEIL DEGRASSE TYSON: Excellent!
Most of the nearly 3,000,000 miles have come from 44 years of crisscrossing the country. The odometer turns over every hundred-thousand miles. Do the math, it's turned over...
IRV GORDON: Twenty-seven times; it's on its 28th.
NEIL DEGRASSE TYSON: So, what does Irv do to get his car to live forever? Well, number one, regular maintenance.
IRV GORDON: I just do the things it says to do, when it says to do them.
NEIL DEGRASSE TYSON: Things like regular tune-ups and oil changes. Irv figures he's gone through 110 tires, 440 spark plugs, 788 oil filters and 3,143 quarts of oil!
Sounds like there could be some lucrative endorsement deals.
So they put you on the payroll?
IRV GORDON: I'm still waiting for my first box of oil filters.
NEIL DEGRASSE TYSON: Tip number two: if something's broke, fix it. Replace the worn-out parts.
Old cars drip oil. How much do you just drip out of this car?
IRV GORDON: You can go underneath my car with a rag, and you won't find any oil.
NEIL DEGRASSE TYSON: Really.
I don't see anything dripping.
IRV GORDON: Bone dry.
NEIL DEGRASSE TYSON: That's because, over the years, Irv has placed his car into the hands of an elite few.
IRV GORDON: This is my A-Team here.
NEIL DEGRASSE TYSON: Richie Vermont serviced the car, from when it was brand new, until he retired seven years ago. He's replaced three clutches and countless brakes and mufflers.
That sounds like it popped.
Irv's maintenance bills helped pay for the education of Richie's kids.
RICHIE VERMONT (Auto Mechanic): I tell you, I sent my kids to college.
NEIL DEGRASSE TYSON: The engine has been rebuilt twice, first by Richie, after 680,000 miles, and in 2009, by this man, Duane Metejka.
All right, what do we got here?
He said it was in pretty good condition.
Oil pump.
IRV GORDON: There was nothing wrong with the oil pump, but I figured after 2,000,000 miles it's a good idea to put a new one in.
NEIL DEGRASSE TYSON: Another rule of thumb?
BOB (Auto Mechanic): Every 2,000,000.
NEIL DEGRASSE TYSON: Obviously you've replaced pieces of this engine. All right, does that allow it to count as the original car, even though you're replacing the parts that wear out?
IRV GORDON, RICHIE VERMONT, DUANE METEJKA, BOB: Yeah. Sure. Un huh. Mmm hmmm.
NEIL DEGRASSE TYSON: Irv says it's not any different from a living organism.
IRV GORDON: Like your body replaces parts. How many times have all those different cells replaced themselves completely, from beginning to end? Does that make you not you? It's the same argument.
NEIL DEGRASSE TYSON: And of course Irv would know.
IRV GORDON: Well, this is coming from a science teacher.
NEIL DEGRASSE TYSON: And so, Irv will keep on going.
IRV GORDON: Who would expect how a car would change your life.
NEIL DEGRASSE TYSON: When you look at pictures taken over the years, you see a man getting older as his car remains as new as the day he drove it off the lot, more than four decades ago.
IRV GORDON: It doesn't show any signs of giving up. And hopefully this second rebuild will outlast my ability to keep driving.
NEIL DEGRASSE TYSON: And that leaves me with one last question: Can I drive your car?
IRV GORDON: Absolutely not. Nobody drives my car but me.
NEIL DEGRASSE TYSON: Nobody?
IRV GORDON: Nobody.
NEIL DEGRASSE TYSON: Nobody?
IRV GORDON: Nobody.
NEIL DEGRASSE TYSON: Nobody?
IRV GORDON: Nobody.
NEIL DEGRASSE TYSON: If something in your car breaks or stops working, like your radiator, you can always just take it out and replace it, but what about us? If my body parts break down, like my heart, I might be able to get a transplant, but right now, even if I could find a replacement part, one, it's going to be used, and two, my body might just reject it. The dream would be to replace my heart, or whatever's broken, with a brand new version, in perfect, working condition, but exactly like my original. People have been talking about this for years, but now, thanks to some brand new discoveries, the dream of custom-made, personalized body parts may soon become a reality.
In the 2005 sci-fi thriller, The Island, people have found a way to live forever: they grow clones and harvest their organs. But real science may be on the verge of a less diabolical solution.
This, for example, is no special effect. It's a lab-grown lung, no clone attached.
DORIS TAYLOR: I absolutely see a day where you'll walk into a manufacturing facility somewhere, and there will be jars of kidneys, jars of livers and jars of lungs, whatever it is you need.
NEIL DEGRASSE TYSON: Just as in The Island, your body would accept the new organ because it would be yours, grown from your cells.
JOSEPH VACANTI (Massachusetts General Hospital): And there would be no more waiting lists for organs, there would be no more rejection. We would enter a new era, where we could build you an identical, ideal replacement.
NEIL DEGRASSE TYSON: But how do you make an organ without a body to build it in?
We've been growing cells in the lab for decades, but they just sit around in flat layers or clumps. So how would you coax them to form a three-dimensional organ like a heart, with chambers, valves and blood vessels?
Maybe it's the same way you go from this to this.
See, an organ is not unlike a building. It's a collection of parts that has to come together and work together. You can think of a cinder block as a cell. The problem is a block or a cell alone is not enough. To construct a building you need to begin with an internal framework, or scaffold, to define the parts and hold them together.
Thirty years ago, transplant surgeon Jay Vacanti and chemical engineer Robert Langer realized that to build an organ, cells also need a framework, a scaffold to guide their growth. The challenge was to engineer scaffold materials living tissue could grow on.
ROBERT LANGER (Massachusetts Institute of Technology): So this is a material that we call "bio-rubber."
NEIL DEGRASSE TYSON: Bio-rubber; and you use the prefix "bio," because whatever is the material, it will take to flesh or living cells?
ROBERT LANGER: That's right.
NEIL DEGRASSE TYSON: So why does the cell even care?
ROBERT LANGER: Because a, because of a lot of things could be toxic to a cell, or the cell wouldn't like their surface and wouldn't be able to grow on it.
NEIL DEGRASSE TYSON: Picky cells.
ROBERT LANGER: Cells are picky, and some are more picky than others.
NEIL DEGRASSE TYSON: But sculpting a scaffold out of the right material was only a start. To turn one into a living body part, an ear, for example, it must then be seeded with cells.
A few weeks in an incubator allows those cells to multiply, covering the scaffold. Then comes a rather strange test.
This is really creepy. I mean, mice are creepy enough, and this one has no hair and a human ear growing on its back.
JAY VACANTI: Yes.
NEIL DEGRASSE TYSON: He doesn't seem to mind that he has an ear growing on his back.
JAY VACANTI: No, he knows he's here for a bigger purpose. But this is a very, very important step in the science, because, on the back of this animal, we're actually incubating and growing perfect cartilage in the shape of a human ear. And it's completely connected to the blood vessels, so that it's just like a native ear in a normal circumstance.
NEIL DEGRASSE TYSON: In the head of a person?
JAY VACANTI: That's correct.
NEIL DEGRASSE TYSON: So when this finally gets implanted in a human you don't expect rejection, as is so common with new body parts.
JAY VACANTI: Exactly, because we're going to start with the patient's own cells, it'll make his own tissue, and, therefore, the body will accept it.
NEIL DEGRASSE TYSON: Within a year, Vacanti and Langer expect to be implanting their ears directly on the heads of soldiers wounded in Iraq and Afghanistan.
But these will not be the first recipients of lab-grown body parts. Already, patients of other doctors have received blood vessels, skin, muscles, even bladders built the same way.
ROBERT LANGER: I think, with enough research, most parts of the body will be replaceable. And I haven't come across very many body parts where somebody, somewhere isn't working on trying to replace them.
NEIL DEGRASSE TYSON: Which is certainly encouraging news for people who need more complex body parts, like 20-year-old Stacey.
STACEY (Liver Disease Patient): I was in the hospital, and that's when they came in and told me that I may need a new liver.
NEIL DEGRASSE TYSON: But will she get one? Every day, nearly 20 Americans die, waiting for donor organs.
JAY VACANTI: So, this problem is an extraordinary problem. There are too few organs for the well-over-100,000 Americans waiting.
NEIL DEGRASSE TYSON: But if we are ever to make the complex organs most needed to save lives, like livers and hearts, the scaffold builders will have to overcome an obstacle, namely, plumbing. In a building it's pretty straightforward. Pipes carry fluid where it's needed, just like blood vessels in the body, except that in a major organ like the heart...
DORIS TAYLOR: You need a blood vessel per cell, because the heart works all day every day. And I don't know if you've ever seen blood vessels, really. But they look like a tree. And the challenge is not to build that big limb, but to build those little tiny branches that come off.
NEIL DEGRASSE TYSON: But building these intricate branches might be unnecessary, if we take advantage of a remarkable fact: organs are not just made of cells.
DORIS TAYLOR: So if you wash the cells away, what's left? And what's left are these proteins on which the cells sit. And they form the framework of the organ, the scaffold.
NEIL DEGRASSE TYSON: These natural scaffolds hold an organ's shape down to the smallest detail, including every blood vessel. So could they be used to build a complex organ like a heart?
Six years ago, no one could say, because no one had ever stripped a heart of its cells, leaving the scaffold intact. But Taylor's colleague, Harald Ott, thought he could find a way. He would use the blood vessels in a rat's heart to deliver a chemical that would dissolve its cells, and nothing else. But which chemical?
HARALD OTT: So the process of finding the right chemical was literally a trial and error process, starting from A to Z on the chemical shelf.
NEIL DEGRASSE TYSON: First, Ott tried enzymes, but they dissolved both the cells and the scaffold. Other chemicals caused the hearts to swell up. Finally, he tried a soap commonly found in shampoos.
HARALD OTT: We saw the heart become translucent. And it was obvious to us all that something had happened that hadn't happened in the months before.
DORIS TAYLOR: What we had is this thing that looked like a heart, but it looked like a ghost heart, if you will.
NEIL DEGRASSE TYSON: Injections of dye showed the scaffold to be undamaged, down to the smallest blood vessels. And we now know that this technique works with many organs, including human-sized ones.
DORIS TAYLOR: This is essentially the scaffold of a heart. Who knew a heart had a full skeleton? But it essentially has no cells, dead or alive. It's beautiful. You can see the blood vessels here, the chambers of the heart. You can see the valves.
NEIL DEGRASSE TYSON: But could a bare scaffold, once again become the framework of a living heart? Taylor soon discovered it was more than a matter of injecting cells.
DORIS TAYLOR: Just putting cells on a scaffold isn't enough. It's putting cells on a scaffold and giving them an electrical signal, and giving them a mechanical blood pressure, and then giving them oxygen. It's not just a heart in a jar. It's a heart in an artificial body. So, it's simple in many ways, and it's unbelievably complicated.
NEIL DEGRASSE TYSON: After eight days, the first lab-grown heart beat on its own.
DORIS TAYLOR: It really makes you go, "What is life?" the first time you see something beat that was dead. It's one of those "yes" moments in life.
NEIL DEGRASSE TYSON: Since then, Ott has joined Massachusetts General Hospital and used the same method to build a pair of lungs. After coming back to life, one lung was successfully implanted in a rat.
So, if you can make a working living lung, then it seems to me that you can...
HARALD OTT: ...build, literally, any organ.
NEIL DEGRASSE TYSON: Any organ!
This novel approach has already made a difference in the real world. In Barcelona, Spain, this woman, Claudia Castillo, might be dead without it.
Two years ago, tuberculosis devastated her windpipe, making it difficult to for her to breath. But surgeon Paolo Macchiarini saw a solution: give Claudia a new windpipe, which her body would never reject, because it would be made of her own cells, grown on a natural scaffold.
And so, in June of 2008, Macchiarini and an international team of specialists removed a windpipe from a human cadaver, washed it clean, and reseeded it with living cells from Claudia's body.
Four days later, the new windpipe was transplanted into Claudia.
PAOLO MACCHIARINI (USP Instituto Universitario Dexeus): If you transplant an organ without tissue engineering, you need immunosuppression, you need close watching. And this was absolutely not the case for Claudia. She never had any sign of rejection. Indeed, four days after surgery she was home.
NEIL DEGRASSE TYSON: More than a year later, Claudia is living a normal life, free of the fear that she will reject her new body part.
CLAUDIA CASTILLO (Windpipe Transplant Recipient/Translation): I feel like the transplant is not from the body of another person. It's mine.
NEIL DEGRASSE TYSON: That sense of ownership might soon be crucial to organ recipients, because their scaffolds might not come from a person at all.
DORIS TAYLOR: This is a pig kidney, sliced in half, and it's the same size, same complexity as a human kidney. We could cover this with human cells and, in theory, build you a kidney.
NEIL DEGRASSE TYSON: Human organs built on natural or artificial scaffolds, made from a patient's own cells to avoid rejection, available in unlimited supply? Most researchers believe it will be a reality within decades, and Taylor is even more optimistic.
DORIS TAYLOR: Kidney, liver, lung...we're not decades away from building something complicated, we more like years away.
In addition to organ scaffolding... there is:
(Text of Tao Xu's paper about Inkjet Gene Printing)
Yes!
Researchers at Wake Forest University adapted an ordinary inkjet printer to print organs.
They filled an empty ink cartridge with cells.
And printed out a two-chamber mouse heart.
Coolest part? It actually beat.
NEIL DEGRASSE TYSON: (As an auto mechanic) Just like some well-made cars, some people last longer than others. They don't fall apart, and they don't even need replacement parts. What's up with that? (As a medical patient) You know, if medical researchers figure it out, maybe everyone could last longer. (As an auto mechanic) Correspondent Ziya Tong tracked down some lucky folks who don't age like most of us and the doctors who are trying to figure out the secret to their Fountain of Youth. (As a medical patient) So how'd I do?
ZIYA TONG: (Correspondent): Some people are like forces of nature: aging gracefully is simple for them.
CHUCK YOGI (Honolulu Heart Program Participant): My name is Chuck Yogi. I'm 91 years old, ever since the last couple of days. So, I'm fully 91.
ZIYA TONG: Somehow, James Harai got to 91, too.
JAMES HARAI (Honolulu Heart Program Participant): And I've been blessed with, uh, good health, I guess, you know?
ZIYA TONG: Kind of makes you wonder how they do it.
Do you have a secret to looking so young at 90?
SAMUEL HARANO (Honolulu Heart Program Participant): Just don't worry about unnecessary things, you know? I'm happy-go-lucky.
ZIYA TONG: But while these guys are living proof that longevity comes naturally for some, other people are pulling out all the stops to try and live as long as they possibly can.
Computer scientist and inventor Ray Kurzweil takes 150 pills every single day.
RAY KURZWEIL (Kurzweil Technologies, Inc.): That might sound like a lot, but it's not enough to just be natural. I take 400 milligrams a day of resveratrol, a lot of vitamin D.
ZIYA TONG: So what's he doing with all those pills?
RAY KURZWEIL: In my view, death is a great robber of all the things that give meaning to life. It destroys knowledge and wisdom and relationships, and there's actually a lot that you can do to slow down these aging and disease processes.
ZIYA TONG: But is Ray wasting his time looking for a Fountain of Youth that's just a myth?
RAY KURZWEIL: The goal, right now, is to live long enough to get to a future point where we will have technologies that will extend our longevity even further.
ZIYA TONG: In fact, scientists have been tinkering in the lab, trying to extend life for a long time, and they've come up with a couple of things that do work in animals. Calorie restriction, for instance, basically putting an animal on a diet, seems to kick in a survival response and helps it live longer. And they've found a substance in red wine that has a similar effect. But what if somebody could figure out how these guys did it so effortlessly?
JAMES HARAI: The fish not cooperating today. I think they're camera shy, I mean.
ZIYA TONG: Cynthia Kenyon thinks she may have found one of the keys to a long life in a tiny, nearly microscopic worm called C. elegans.
So how can we learn anything about human aging from these tiny little worms?
CYNTHIA KENYON: I know they look really different from us, but the basic processes of life are very similar at the molecular level.
ZIYA TONG: The good thing about these little guys is that they get old and die in just a little over two weeks.
CYNTHIA KENYON: Okay, watch this. I'm going to show you something really cool, now.
So, this is the normal worm when it's young.
ZIYA TONG: So that's a nice, sprightly worm; what I'd expect a worm to look like. Quite fiesty!
CYNTHIA KENYON: Now, I'm going to show you the same kind of worms, but just two weeks later, when they're old.
ZIYA TONG: Wow!
CYNTHIA KENYON: So this is a, yeah, normal worm when it's old. You can see that they're about to die.
ZIYA TONG: Oh, wow. So these are really slow-moving here. I didn't think you could see aging in a worm so dramatically.
CYNTHIA KENYON: Okay, so now what I'm going to show you are worms that are the same age, but you'll see that they look much younger.
ZIYA TONG: So these worms are the exact same age as the ones that we saw that were almost dead?
CYNTHIA KENYON: Yup, they look much younger, even though they're the same age.
ZIYA TONG: And they're wriggling about just like the other ones, huh?
CYNTHIA KENYON: So they're like 90-year-old people who look 45.
ZIYA TONG: That's incredible. So what's different about these?
CYNTHIA KENYON: We've changed one gene; that's all.
ZIYA TONG: Kenyon changed one gene in the worm. Genes are made of D.N.A., long strings of 4 chemicals, best known by their initials: A, G, C and T. Together, they form the basis of all life on Earth. Kenyon found that there was a gene that scientists call FOXO, which had a central role in keeping her worms freakishly youthful.
CYNTHIA KENYON: What FOXO does is it helps the animal to protect and repair its tissues. The reason that it can do it is this one gene controls a lot of other genes.
ZIYA TONG: FOXO is a master control gene, meaning it regulates hundreds of other genes, genes that have a profound effect on the worms' health.
CYNTHIA KENYON: So you can think of it as a superintendent of a building. So if you have a building, a nice big building, obviously it has to be maintained. What FOXO does, or the building superintendent does, is to keep the building in good working order.
ZIYA TONG: The superintendent makes sure that the electricity works and that the roof doesn't leak.
CYNTHIA KENYON: It makes sure that the walls are painted, by hiring painters; it makes sure that the floors are swept.
ZIYA TONG: But the superintendent doesn't actually do all these important jobs.
CYNTHIA KENYON: The building superintendent would hire workers to do these different things. What FOXO does, in the cell, is it switches on other genes.
ZIYA TONG: Those worker genes do jobs like enhancing the immune system and protecting the cells from bacterial infection.
CYNTHIA KENYON: Some of these genes that protect the cell make proteins that will kill invading micro-organisms. Others are switched on that are antioxidant genes.
ZIYA TONG: Kind of like a rust inhibitor for a cell.
Now, most living things need oxygen, but oxygen can actually be damaging to cells that aren't prepared to deal with it. And, yes, there's a worker gene for that, too.
CYNTHIA KENYON: I'd say, altogether, there are probably about a hundred worker genes that have very important roles. And, together, what you get is a cell or tissue or an animal that stays in really good working condition for a lot longer.
ZIYA TONG: All those processes are actually directed by the FOXO superintendent gene.
Kenyon tweaked one gene in the worms and made FOXO more active. With a more active superintendent, the cells became more resilient than normal and Kenyon's worms lived twice as long.
If there's one gene that dramatically increases lifespan in worms, could the same be true in humans?
JAMES HARAI: I went to Alaska 10 times.
ZIYA TONG: Yeah? They have big fish in Alaska, right?
Mr. Harai and the others are part of a groundbreaking 45-year study in Hawaii that's trying to find out.
BRADLEY WILLCOX, M.D. (Kuakini Medical Center): The Honolulu Heart Program population is a group of Japanese-American men...
SAMUEL HARANO: Beautiful sunset...
BRADLEY WILLCOX: ...that we have followed since the 1960s.
CHUCK YOGI: When you hear people my age, they say it's so hard to even get out of bed, so I say, "So why don't you jump up?" But they say, "No, no!"
ZIYA TONG: What's he have that other people don't?
CHUCK YOGI: Thirty-five times.
BRADLEY WILLCOX: What's important for aging is it's a process. So we've studied the process in these men for decades.
ZIYA TONG: Willcox and geneticist Timothy Donlon wanted to see if they could find out anything about the genetics of human aging from this unique scientific resource.
TIMOTHY DONLON (Kuakini Medical Center): This is one of the freezers that houses the over 8,000 samples from this project that's been conducted over the last 45 years.
ZIYA TONG: Wow. So this is, like, data, frozen in time?
TIMOTHY DONLON: That's right, safely tucked away, here.
ZIYA TONG: Using these samples, they tested five genes that had already been shown to help animals live longer, to see if any of them would extend human life as well.
BRADLEY WILLCOX: And based on that list, we found one gene that was heads and shoulders above everything else. And that was the FOXO gene.
ZIYA TONG: The FOXO gene! That's right: the same superintendent gene that helped double the life of Cynthia Kenyon's tiny worms. Though everybody has the FOXO gene, these Hawaiian men seem to be living longer, healthier lives because they have a protective version of FOXO.
TIMOTHY DONLON: We found that if you have this FOXO gene, you have a two-fold chance of living to a hundred. And if you have two copies of this, you have a threefold chance of living to a hundred.
ZIYA TONG: A gene typically consists of two copies. You get one copy from your mother and one copy from your father.
BRADLEY WILLCOX: So with FOXO, the area that we looked at, you could have a C or a G from your mom and your dad. The vast majority of us have two Cs. About 25 percent of us have one G and one C, and about 10 percent have two Gs. If you have two Gs, you hit the jackpot: that's triple the odds of living to be a hundred. You can go to Vegas with those odds!
ZIYA TONG: I'm not very good at this, but I read palms a little bit, and, believe it or not, you actually have an incredibly long lifeline.
SAMUEL HARANO: No kidding?
ZIYA TONG: Yeah, you do!
BRADLEY WILLCOX: And not only triple your odds of living that long, but being healthy. So it was a gene that appeared to be associated with extended health-span, not just lifespan.
CYNTHIA KENYON: It tells us that FOXO in humans affects aging. You could have imagined that we have the gene, but it doesn't do the same thing, but this says it does!
ZIYA TONG: News of the Hawaii study sped around the world, and scientists confirmed the results in population after population: in Germany, Italy, New England, California and in China.
Nir Barzilai of the Albert Einstein College of Medicine in New York...
HAROLD LAUFMAN (Jewish Centenarian): I'm now 98 years old.
ZIYA TONG: ...also found a similar pattern in the FOXO genes of Ashkenazi Jewish centenarians.
WOMAN (Jewish Centenarian): I'm 96.
MAN (Jewish Centenarian): Ninety-seven.
WOMAN (Jewish Centenarian): Ninety-eight.
NIR BARZILAI (Institute for Aging Research, Albert Einstein College of Medicine): This data on the FOXO pathway that came from Hawaii and then confirmed by us, was confirmed by other groups. And, in fact, it's the most consistent, validated study in this field, suggesting that this is real and important for human aging and longevity.
ARTHUR STERN (Jewish Centenarian): We don't feel old; we feel young.
ARTHUR STERN'S FRIEND (Jewish Centenarian): We don't feel old.
NIR BARZILAI: And it's also consistent with what we have learned, that there's this whole concept of a superintendent that is regulating whatever is going in the house.
ZIYA TONG: Oh! You got one, you got one, you got one!
And in the future, that knowledge could be used to develop new drugs to combat age-related diseases...
JAMES HARAI: Fish on!
ZIYA TONG: ...and, perhaps someday, to help us live longer.
Good job, Mr. Harai!
BRADLEY WILLCOX: The vast majority of us get an average set of genes. So it's what you do that becomes most important: eating a good diet, regular physical activity, engaged in life.
CHUCK YOGI: As you age, I think every little thing pleases you more than in the past.
SAMUEL HARANO: And now I've got to aim for the century mark, yeah?
ZIYA TONG: So how do you think you're going to celebrate your 100th birthday?
SAMUEL HARANO: Hundred candles? It would be a fire hazard, huh?
ZIYA TONG: Yeah, it would be a fire hazard.
Meet the jellyfish Turritopsis dohrnii.
They begin life as a larva...
Then turn into a "polyp"...
And then become a mature jellyfish.
But when times get tough, it can revert to a polyp again!
Then later grow back into an adult jellyfish.
It can live FOREVER.
In theory.
NEIL DEGRASSE TYSON: (As an auto mechanic) At least for now, it's much easier to extend the life of a car than of a person like you or me. The car's not flesh and blood and complex organs. But what if you could create a version of yourself that was indestructable?
In this episode's profile, we'll meet a computer scientist who wants to build virtual versions of ourselves, avatars, that look, act and talk like real people and who will hang around, long after the flesh and blood versions of us are dead and gone.
In 1987, when Jason Leigh tuned in to Star Trek: The Next Generation, he saw the holodeck for the very first time:...
BRENT SPINER (as Lieutenant Commander Data, Star Trek: The Next Generation/Film Clip): I was curious to see how three of history's greatest minds would interact in this setting.
NEIL DEGRASSE TYSON: ... a place where people from the distant past can live on as computer-generated holograms.
BRENT SPINER (as Lieutenant Commander Data, Star Trek: The Next Generation/Film Clip): End program.
NEIL DEGRASSE TYSON: And that's when a sci-fi TV show gave this computer scientist his big idea: a way to allow all of us to, in a sense, live forever.
JASON LEIGH: If you were to think about that when Star Trek first came out, you would think, "Oh, this would be impossible to do," but now it's possible.
NEIL DEGRASSE TYSON: At the University of Illinois, Chicago, Jason has been obsessed with turning this fantasy into reality through Project Lifelike, a plan to make immortality available to anyone by creating a virtual copy of you as an avatar—a concept that intrigued Jason, long before James Cameron parlayed it into a billion dollar blockbuster.
JASON LEIGH: An avatar is an instance of yourself that's digital, that will never die.
NEIL DEGRASSE TYSON: Jason knows he can't really make you live forever, but he can use computers to preserve your thoughts, memories and even the way you look, for eternity.
JASON LEIGH: Can I live forever?
JASON LEIGH'S AVATAR: In the future your children's children will be able to meet with you. Students will be able to talk to scientists long gone, like Steven Hawking or Neil deGrasse Tyson, even.
NEIL DEGRASSE TYSON: Who, me? Well, I'd be honored.
Jason's vision of a world where we can build relationships with dead people, from the famous to family members, was deepened by another movie.
JASON LEIGH: In the film Superman, Jor-El, who is Superman's dad, is long-gone and dead.
MARLON BRANDO: (As Jor-El, Superman/Film Clip):You do not remember me. I am your father.
JASON LEIGH: But his dad's able to counsel him, as if he were still alive.
NEIL DEGRASSE TYSON: Jason's journey began in 1960s Hong Kong, where he was a shy, awkward boy, with no real friends.
JASON LEIGH: I was really, I guess a "geek" would be a good term for it, but I was also artistically inclined. And I was drawing anything I saw in science fiction.
ACTOR'S VOICE (Star Trek/Film Clip): It's a missile, and it's heading straight for us.
JASON LEIGH: Star Wars blew my mind: all these wonderful and cool technologies that we, as mere mortals living today, didn't have access to.
NEIL DEGRASSE TYSON: And before long, Jason was drawing his own inventions.
JASON LEIGH: I remember we had these three-ring binders and paper would always rip, and it just drove me nuts. And so I was imagining some futuristic computer. It wasn't a computer then—I didn't know what a computer was—a futuristic magical pad, where I would write on. Of course, nowadays we call that a tablet computer.
STEVE JOBS (Apple/File Footage): And we call it the iPad!
JASON LEIGH: If only I'd patented it back then.
NEIL DEGRASSE TYSON: Then Jason saved up to buy his own computer. And he instantly became obsessed, spending nine hours a day at the keyboard.
JASON LEIGH: Even when I went to sleep, I was writing code, while I was asleep. And I would find an error, and I would wake up, and I would find, yep, certainly there was an error in the code.
NEIL DEGRASSE TYSON: When Jason left Hong Kong to go to college, he had promised his parents he would study the well-established field of chemical engineering, but he had a secret plan.
JASON LEIGH: The first day I landed in the U.S., I head straight for the computer science department and said, "How do I switch majors?" And then I wrote a letter back to my dad and said, "I switched to computer science." He was actually supportive.
NEIL DEGRASSE TYSON: After college, Jason was eager to make his mark designing computer graphics, and he learned about E.V.L., the Electronic Visualization Lab, in Chicago.
JASON LEIGH: It was people who had long hair, all sorts of strange and crazy people.
NEIL DEGRASSE TYSON: These self-proclaimed "techno-hippies" were finding new ways to merge computers and art.
JASON LEIGH: And I thought, "Wow, finally, a program that thinks and does things the way I've always wanted to do." I said, "Well, I'm going to just let my hair grow out." So I fit in and became one of the techno-hippies.
So I think it's a great time to be a geek.
NEIL DEGRASSE TYSON: At E.V.L., Jason blends games and movies into every aspect of his life, from his work to his play, to his car and even his kendo, which is as close as he's going to get to a lightsaber battle in Chicago.
JASON LEIGH: It very much is bringing Jedi knight-ism into reality.
No respectable Jedi knight would use any other Jedi's sword.
NEIL DEGRASSE TYSON: After 15 years, Jason became lab director, and he could now take aim at his most ambitious sci-fi fantasy: how to create a realistic avatar.
Still an artist at his core, Jason used art as inspiration and his drawing skills to develop ideas. In 2007, Jason joined the growing field of avatar researchers, as he began work on his plan to live forever. As usual, Jason started with a drawing.
JASON LEIGH: I always start with a picture in my mind. The picture goes onto paper. The picture goes into the computer. I spin the thing around to see if it makes sense.
NEIL DEGRASSE TYSON: Next, Jason had to make an avatar look like a living, breathing person. For a guinea pig, he used himself.
JASON
LEIGH: First
of all, we take photographs of their face from multiple angles, so that we can
use software to reconstruct the face in three dimensions, as realistically as
we can.
NEIL DEGRASSE TYSON: Then Jason teaches his double to move just like he does.
JASON LEIGH: And so, for that, we put them in a motion-capture suit.
NEIL DEGRASSE TYSON: Jason even records and modifies his avatar's emotional expressions.
JASON LEIGH: Like, whether they were happy, sad, angry.
Let's look at a little bit of anger. There you go.
NEIL DEGRASSE TYSON: As Jason fine-tuned the graphics, he needed to teach his avatar to think and talk. So he turned to artificial intelligence experts.
JASON LEIGH: Our collaboration involves researchers in Florida.
NEIL DEGRASSE TYSON: To overcome the thousand-mile gap between collaborators, Jason's team invented their own 20-foot video wall, so the researchers in Chicago and Florida could work on the avatar's intelligence as if they were in the same room.
FLORIDA RESEARCHER(On Video Conference): The avatar doesn't use its hands a lot to talk, and I think at some point, they need to show...
JASON LEIGH: Yes.
FLORIDA RESEARCHER (On Video Conference): ...some hand-waving motion.
NEIL DEGRASSE TYSON: Jason records the ideas and thoughts he wants his avatar to be able to express...
JASON LEIGH: Designing computer algorithms is like writing poetry or painting a picture.
NEIL DEGRASSE TYSON: ...so that someone can sit down ask his avatar dozens of questions.
JASON LEIGH: Why is Star Trek important to you?
JASON LEIGH' AVATAR: Star Trek portrayed so many compelling ideas about our future.
NEIL DEGRASSE TYSON: And this idea of preserving our life experiences for future generations has been catching on.
JASON LEIGH: When I watched Avatar, the most interesting notion about it was when these people passed on, their knowledge is absorbed into this tree of past knowledge. And I thought, "Aha! That's what we're trying to do."
Ultimately, what you have is a collective knowledge of people.
NEIL DEGRASSE TYSON: Jason dreams of a future where anyone can program all of their thoughts, feelings, memories, hopes and fears into a virtual replica of themselves, so people can actually speak directly to those long-gone.
JASON LEIGH: What we'd like to do in the future is to try to break the avatar out of the box, make it a person in the real world, conversational avatars that are as intelligent as humans.
JASON LEIGH'S AVATAR: You mean me? You mean I'm not really alive?
Why are they called avatars?
In Hinduism, an Avatara is a divine being that takes the form of a human or beast.
Avatara literally means crossing down to the realm of humans.
Avataras support the pious, annihilate the impious and reestablish righteousness.
And unlike Jason Leigh's "avatar"...
They are mortal.
NEIL DEGRASSE TYSON: (As a driver) Most things that break in a car can be fixed, but every now and then, some things can go catastrophically wrong and the results could be fatal.
(As an auto mechanic) In those situations, if we could freeze time, we could fix the problem before the worst happens.
(As a driver) Well, some doctors think they can do just that with people who are in the middle of life threatening crises like a heart attack or stroke. Correspondent Peter Standring found out how freezing, or at least slowing down time, is already saving lives. (As an auto mechanic) All set. Drive safely. (As a driver) Thank you!
PETER STRANDRING (Correspondent): Every once in a while there are news reports of miraculous survivals that seem almost too incredible to be true: people who drown in icy water or are buried in snow; their hearts stop beating and they're getting no air; they seem to be dead, yet, mysteriously, they come back to life. Somehow, their bodies seem to go into a state of suspended animation, so they can survive without oxygen for an hour or more, instead of mere minutes. But how?
Researchers have been looking for clues in some surprising places, starting with this guy: the thirteen-lined ground squirrel. He might not look like he has much in common with near-death survivors, but, in fact, he's an expert at surviving an experience that seems like it should kill him: hibernation.
Today, I've come to Minnesota in search of these hibernating squirrels, and it's freezing.
So the squirrels are actually underneath all of this snow and under the ground, right here?
MATT ANDREWS (University of Minnesota): All around here. The animals are probably about four feet below the surface of the snow.
PETER STRANDRING: Man, how do they survive that?
MATT ANDREWS: That's a great question, and it's something we're trying to figure out in the laboratory.
PETER STRANDRING: Matt Andrews is trying to unlock the secrets of hibernation. Could the hibernating squirrels have something in common with people who mysteriously come back from the dead?
So what is this place? What is this room, Matt?
MATT ANDREWS: This is the environmental chamber where we keep our animals in a state of hibernation.
PETER STRANDRING: Why are we whispering?
MATT ANDREWS: We want to duplicate the conditions that the animal experiences underground during the winter, and so we're duplicating that here, in the laboratory setting.
PETER STRANDRING: The squirrels' dark laboratory home is kept at 40 degrees, about the same as it would be underground.
So, underneath all this sawdust we have our thirteen-lined ground squirrel?
MATT ANDREWS: This is exactly what they would look like when they were in their burrow. And there is a hibernating thirteen-lined ground squirrel.
PETER STRANDRING: Comfy, cozy, rolled up in a ball.
MATT ANDREWS: And this is the way this animal spends the winter. It can survive in this state for months. Would you like to hold one?
PETER STRANDRING: Absolutely. Wow. Look at that: my own little bundle of fur. I think it's a first for me, holding a hibernating animal.
When these squirrels go into hibernation, it's an amazing process. Their heart rates drop from 300 beats per minute to three or four. Their body temperature drops from about 98 degrees to about 40. They only take a few breaths a minute and use barely two percent of the amount of oxygen they need when they're awake.
Now, you might think that all those drastic changes would be enough to kill off these little guys, but despite it all, they emerge from their long winter slumbers completely fine. There's no damage to any of their organs. There's no damage to their brains. It's really incredible.
MATT ANDREWS: Okay, it's time to put him back to bed now.
PETER STRANDRING: Okay, make sure you tuck him in good.
Hibernation is deeply mysterious.
MATT ANDREWS: So, Ann, what do you have going on here?
PETER STRANDRING: In his lab, Matt Andrews studies its impact on genes. He's discovered several genes that get turned on in some cells only during hibernation. He hasn't completely solved the mystery, but one thing is clear: somehow, those genes seem to reduce the hibernating squirrels' need for oxygen.
Andrews' ultimate goal is to figure out how we could do the same for people.
MATT ANDREWS: If you can understand the molecules that are expressed when an animal hibernates, you can, possibly, develop a therapy that can mimic the hibernation experience, so that a person can survive a traumatic injury: a heart attack, a stroke, those sorts of things.
PETER STRANDRING: Heart attack victims usually die from a lack of oxygen, but if we could reduce the body's need for oxygen, even temporarily, who knows how many lives could be saved. While the squirrels do it with their genes, those drowning and avalanche victims who come back from the dead somehow appear to survive without oxygen because of the cold.
Now, at a handful of hospitals around the country, emergency room doctors are attempting to replicate those miraculous recoveries.
Todd Van de Bussche is living proof it can work. Todd was just 39 years old when one day he collapsed in the shower with a sudden cardiac arrest.
BETH VAN DE BUSSCHE (Todd Van de Bussche's Wife): All of a sudden, Todd fell over and then he stopped breathing.
PETER STRANDRING: When the paramedics arrived, Todd was technically dead. Luckily, he was brought to this E.R. in Virginia, where doctors are trying out a new treatment.
E.R. STAFF PERSON: It sounds like E.M.S. got a pulse back in about 20 minutes.
PETER STRANDRING: While one team worked frantically on Todd's heart, another flooded his bloodstream with a solution of icy fluids and drugs.
JON ORNATO (Virginia Commonwealth University): By cooling as quickly as possible, we're trying to lower the body's metabolism; we're trying to lower the rate at which the body consumes and burns up oxygen.
E.R. STAFF PERSON: You're going to have to wait till we get these tubes in. We're hurrying as fast as we can.
PETER STRANDRING: As the cold fluid flooded his veins, Todd's body temperature dropped from 98 degrees to about 92. His heart-rate slowed, and all the cells in his body used a fraction of their normal oxygen.
Much like the hibernating squirrels', Todd's body was carefully put into a state of suspended animation.
JON ORNATO: We're trying to stretch time, to give the body a chance to recover from the cardiac arrest.
PETER STRANDRING: Twenty-four hours later, Todd was slowly warmed up and brought back to life. Now, two years later, he's in good health and enjoying life with a new baby.
JON ORNATO: We're seeing that some of the patients that years ago we thought could never survive are now waking up and going back to a fully functional existence.
PETER STRANDRING: This cooling therapy is still relatively new, but, in the few places it's been tested, it's substantially increased survival rates for some kinds of heart attacks. And Todd has had one of the best recoveries so far.
TODD VAN DE BUSSCHE: I've gone through it, and look how well my outcome has been. It's truly a miracle.
NEIL
DEGRASSE TYSON: And now for some
final thoughts on living forever. The urge to not want to die is as natural as
life itself. But you should always be careful what you wish for. One day, it
just might come true. If, starting now, everyone in the world lived forever,
then Earth's current population of seven billion, which would, at its current
rate of growth, double in 60 years, would instead double in only 35. Take this
forward six centuries or so, and you have so many people on Earth that
everybody will have to stand up straight, just to fit on all the world's land
area. So that leaves interplanetary colonization as the only obvious next step
to accommodate such vanities. But not all planets are Earth-like. Actually,
none of the known planets, inside or outside our solar system, are Earth-like.
Which means if bio-mechanical genetic engineering is what grants you
immortality, then why not alter or enhance our organs in ways that allow us to
thrive under the exotic conditions of alien planets? And that could only be decades
away. There's just one catch. You need a space program capable of leaving
Earth entirely, rather than just driving round the block in Earth orbit. Until
then, our bodies may out-advance our access to space, making Earth a very
crowded place to come. And that is the Cosmic Perspective.
And now, we'd like to hear your perspective on this episode of NOVA ScienceNOW.
Log on to our Web site and tell us what you think. You can watch any of these
stories again, download additional audio and video, explore interactives, hear
from experts and watch revealing profiles of our Web-only series, The
Secret Life of Scientists and Engineers.
Find it all at pbs.org.
That's our show. We'll see you next time.
Credits
NOVA scienceNOW: Can We Live Forever?
- Edited by
- Vincent Liota
- Written and Produced by
- Vincent Liota
- Body Shop – Body Parts
- Edited by
- Sarah Holt
- Written, Produced and Directed by
- Sarah Holt
- Can We Slow Aging?
- Edited by
- David Chmura
- Written, Produced and Directed by
- David Chmura
Jason Leigh - Edited by
- Jessica Reynolds
- Produced by
- Joshua Seftel & Jesse Sweet
- Written and Directed by
- Joshua Seftel
- Hibernation
- Edited by
- Dick Bartlett
- Written, Produced and Directed by
- Elizabeth Arledge
- Executive Producer
- Samuel Fine
- Executive Editor
- Neil deGrasse Tyson
- Senior Series Producer
- Vincent Liota
- Senior Editorial Producer
- Julia Cort
- Supervising Producers
- Stephen Sweigart
Joey David Jovanovich - Senior Editor
- David Chmura
- Colorists
- David Chmura
Laura Raimondo - Senior Researchers
- Sharon Kay
Alison Snyder - Associate Producer
- Fran Laks
- Graphic Design
- Brian Edgerton
- Compositor & Animator
- Yunsik Noh
- Music
- Rob Morsberger
- Sound Mix
- Bill Cavanaugh, RazorMix, Inc.
- Assistant to Neil deGrasse Tyson
- Elizabeth Stachow
- NOVA scienceNOW series animation
- Edgeworx
- Associate Producers
- Julie Crawford
Alyssa Tucker
Laura Willcox - Camera
- Steve Baum
Austin de Besche
Mark Gunning
Chapin Hall
Gareth Manwaring
Stephen McCarthy
Erik Prentnieks
Erich Roland - Sound Recordists
- Mike Bacon
John Cameron
Tim Korn
Janice Mahan
Mark Skoglund
Ben Steger
Aaron Webster - Animation
- Steve Benjamin
Rob Chapman
Brian Edgerton
Nathan Hendrie
Anthony Kraus - Creative Consultant for Body Shop for Body Parts Segment
- Ethan Herberman
- Prop Maker
- Spencer Anderson
- Production Assistants
- Yolanda de la Rubia
Laura Green
Grayhmn Jennings
Ari Manin
Matt Muege
Nick Nutu
Missy Schlade - Archival Material
- Chris Cassidy/Casspix
Getty Images
Paolo Macchiarini
Joseph Vacanti
Shutterstock
iStockphoto
Lifesaving Resources Inc.
National Oceanic and Atmospheric Administration
Michelle van Vliet, Division of Plastic and Reconstructive Surgery, UCLA Wake Forest University Baptist Medical Center - Special Thanks
- Dom's Auto
Volvoville
Richie Vermont
Duane Matejka
Stacey Avallone
Dr. Anthony Atala
Dr. Sangeeta Bhatia
Erik Bassett
Katherine Kulig
Cathryn Sundback
Teague O'Meara
Kathleen Granchelli
Peter G. Hamill
Michael Hunter
Genzyme Biosurgery
Hiromi Nakada
David Curb, MD
Eva Ardo
Maxine Brown
Paul Francuch
Shaun Gayle
Andrew Johnson
Evolution Time Pieces
Bertram Kalisher
Dr. Lewis Gross, Holistic-Dentists
Turner Construction Company
Kuakini Health System - Neil deGrasse Tyson is director of the Hayden Planetarium in the Rose Center for Earth and Space at the American Museum of Natural History.
- NOVA Series Graphics
- yU + co.
- NOVA Theme Music
- Walter Werzowa
John Luker
Musikvergnuegen, Inc. - Additional NOVA Theme Music
- Ray Loring
Rob Morsberger - Post Production Online Editor
- Spencer Gentry
- Closed Captioning
- The Caption Center
- NOVA Administrator
- Kristen Sommerhalter
- Publicity
- Eileen Campion
Victoria Louie
Karen Laverty - Marketing
- Steve Sears
- Researcher
- Kate Becker
- Production Coordinator
- Linda Callahan
- Paralegal
- Sarah Erlandson
- Talent Relations
- Scott Kardel, Esq.
Janice Flood - Legal Counsel
- Susan Rosen
- Post Production Assistant
- Darcy Forlenza
- Associate Producer Post Production
- Patrick Carey
- Post Production Supervisor
- Regina O'Toole
- Post Production Editor
- Rebecca Nieto
- Post Production Manager
- Nathan Gunner
- Compliance Manager
- Linzy Emery
- Development Producer
- Pamela Rosenstein
- Business and Production Manager
- Jonathan Loewald
- Senior Producer and Project Director, Margret & Hans Rey/Curious George Producer
- Lisa Mirowitz
- Coordinating Producer
- Laurie Cahalane
- Senior Science Editor
- Evan Hadingham
- Senior Series Producer
- Melanie Wallace
- Executive Producer
- Howard Swartz
- Managing Director
- Alan Ritsko
- Senior Executive Producer
- Paula S. Apsell
This material is based upon work supported by the National Science Foundation under Grant No. 0917517. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
NOVA scienceNOW is a trademark of the WGBH Educational Foundation
NOVA scienceNOW is produced for WGBH/Boston by NOVA
© 2011 WGBH Educational Foundation
All rights reserved
Image
- (mouse)
- © Nature Picture Library/Britain On View/Getty Images
Participants
- Beth Van de Bussche
- Matt Andrews
- University of Minnesota
- Neil deGrasse Tyson
- Astrophysicist, American Museum of Natural History www.haydenplanetarium.org/tyson/profile/bio
- Joe Ornato
- Virginia Commonwealth University
- Peter Standring
- Correspondent
Related Links
-

Suspended Animation
Cell biologist Mark Roth says that suspended animation isn't just science fiction.
-
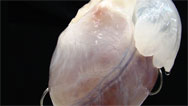
Replacing Body Parts
Custom-made hearts, lungs, kidneys, and other organs could revolutionize organ transplantation.
-

Can We Slow Aging?
A gene called FOXO may be a real elixir of longevity. Can all of us harness its power?
-

Frozen Frogs
The common wood frog freezes solid every winter and then, come spring, defrosts and mates.