
We recommend you visit the interactive version. The text to the right is provided for printing purposes.
|
|
|
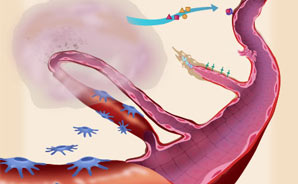
What is "normal" blood vessel growth?
The branching network of blood vessels in our bodies is vast. Lined up end-to-end, the blood vessels in just one adult could encircle the Earth twice. While it's essential to keep this network open and flowing, we rarely need to extend or repair it. In fact, blood vessel growth—or angiogenesis—is a tightly controlled process, occurring primarily at three times: 1) as a fetus develops; 2) during menstruation and pregnancy; and 3) when wounds heal. New vessels also sprout occasionally, in regulated spurts, as babies and children grow. In cancer and other diseases, however, abnormal, uncontrolled angiogenesis spurs the progression of illness. Here, explore how this harmful process unfolds, and learn about strategies to cut it short.—Susan K. Lewis
|
|
|
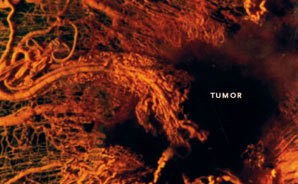
Why is blood vessel growth critical in cancer?
It was one of Judah Folkman's great epiphanies: Without a private supply of new blood vessels, cancerous tumors can't grow larger than the head of a pin and are unlikely to become lethal. Most of us have microscopic cancerous tumors in our bodies at some time, but without blood vessels to feed them oxygen and nutrients, these tumors remain tiny and are unable to spread.
This colored scanning electron micrograph image shows hundreds of chaotically structured blood vessels heading toward a tumor in the background (apparent only as a black void). Find out how such a deadly support system develops.
|
|
|
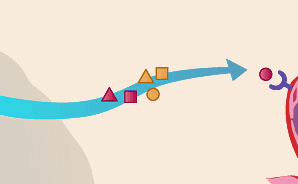
Tumors release molecular signals that recruit new blood vessels.
Triggered by genetic mutations and a shortage of oxygen, tumors release proteins called angiogenic growth factors. In essence, they are molecular "ON switches" that give rise to new blood vessels in various ways. Researchers have identified some 30 different growth factors. The most studied, vascular endothelial growth factor (VEGF), spurs angiogenesis in cancers of the colon and lung, among others. The body also produces many natural "OFF switches" or inhibitors of angiogenesis, such as endostatin. But when "ON switches" overwhelm these natural inhibitors, cancer progresses.
|
|
|
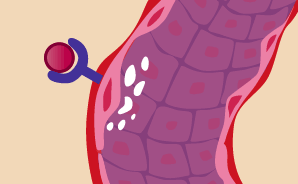
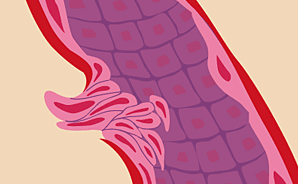
Molecular signals reach existing blood vessels and "turn on" growth.
Once growth factors such as VEGF reach nearby existing blood vessels, they latch onto specific receptors on their surfaces. This sets off another chain of molecular messages. The thin flat cells lining the inside of blood vessels, called endothelial cells, begin making enzymes that dissolve tiny holes in the vessel walls (seen here in white). Researchers are working on ways to stop various growth factors from ever connecting up with receptors. The first FDA-approved anti-angiogenesis drug for cancer, Avastin, binds directly with VEGF to keep it from reaching its target. Similar drugs are in clinical trials.
|
|
|
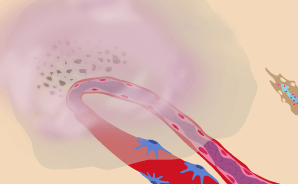
Endothelial cells migrate through holes in the vessel walls.
Once enzymes have cut their way through the outer sheath, or "basement membrane," of the existing blood vessel, the path is open for new blood vessels to sprout. The endothelial cells that have by now started to divide and proliferate migrate through the holes of the existing vessel towards the tumor. Rather than escape entirely, the endothelial cells link together to form the initial scaffolding of new vessels. Researchers are investigating ways to prevent the cells from proliferating and moving outward.
|
|
|
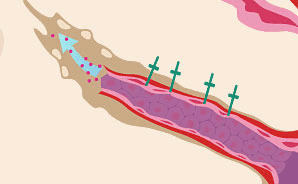
New blood vessels sprout toward the tumor.
As a new vessel sprouts, various molecules aid its progress. So-called "adhesion molecules" (shown as green crosses) help endothelial cells adhere to one another, pulling the sprouting vessel outward, a bit like grappling hooks help an ascending climber. Other molecules called matrix metalloproteinases, or MMPs (shown as red dots), dissolve the tissue in front of the vessel tip, opening the way for it to move toward the tumor. Researchers are exploring ways to hamper MMPs, and drugs that target adhesion molecules have looked promising in cancer studies.
|
|
|
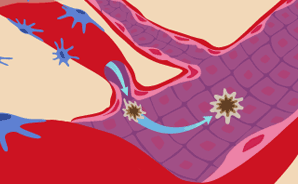
Blood vessel tubes close and form loops.
As the walls of the new vessels build up cell by cell, they undergo shape changes to form tubes. These new tubes then interconnect with one another and make contact with the tumor, merging together to create loops. (This graphic shows just one loop heading into the tumor. A large tumor may have hundreds of capillaries feeding it, and they appear like a tangled web.)
|
|
|
Muscle cells stabilize vessels, blood flows, and cancer can progress.
Finally, smooth-muscle cells called pericytes migrate from existing blood vessels and wrap around the newly formed vessels, stabilizing them. With the squeezing action of these muscle cells, blood flows directly to the tumor, feeding its growth. Now, not only is the tumor able to expand beyond its pinhead size and invade surrounding tissue, but even more dangerously, there is a pathway for metastasis: Tumor cells can dislodge from the initial tumor, move through the bloodstream, and take root in other parts of the body. And these additional tumors will start the angiogenesis process again.
|

|
|
How to stop a deadly process
Angiogenesis is obviously complex, involving dozens of different growth factors that trigger a cascade of subsequent events. Even if a drug effectively blocks one angiogenic growth factor, such as VEGF, blood vessels may still develop via other pathways. As researchers learn more about the various growth factors at play, they are crafting and testing a wider range of anti-angiogenesis drugs in different cancer types.
Currently, doctors are prescribing the first generation of anti-angiogenesis drugs for the treatment of late-stage cancer, often as a supplement to traditional chemotherapy and surgery. As doctors become more experienced with them, anti-angiogenesis drugs will likely be used to treat earlier-stage cancer. Hopefully, patients may one day be able to take anti-angiogenesis therapies much earlier in the progression of disease, with the goal of preventing cancer recurrence or keeping tumors from ever developing in the first place.

|
|
|




