|
|
 |
 |

See the full slideshow
|
| |
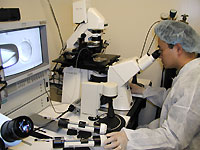
The cloning of embryos for generating stem cells, a process that holds promise for the future treatment of
deadly diseases such as diabetes and Parkinson's, is delicate yet
straightforward. In this slide show showing somatic cell nuclear transfer, as it's also known,
we explain how we clone egg cells to create embryonic stem
cells in our lab at Children's Hospital Boston. Unless otherwise noted, images
show mouse cells.—Kitai Kim & Willy Lensch
Drs. Kitai Kim and M. William Lensch work in Dr. George Daley's laboratory in
the Division of Hematology/Oncology at Children's Hospital Boston. All three
researchers are also associated with Harvard Medical School.
|
| |
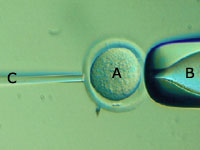
The first step in the process of generating embryonic stem cells is to remove
the nucleus from an unfertilized egg cell (A). Because the egg cell is only 100
micrometers, or one-tenth of a millimeter, wide, we monitor this fine surgical
extraction with a microscope (see previous image). We use a suction pipette (B)
to hold the egg cell steady and a glass needle (C) to remove the cell's
nucleus.
|
| |
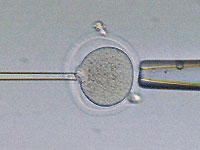
We have gently pushed the glass needle through the tough shell that surrounds
the egg cell. In nature, the zona pellucida, as this shell is known, protects
the egg as it travels down the fallopian tube on its way to the uterus; it also
regulates fertilization so that only a single sperm may enter the egg. Here,
the glass needle is in the process of removing the nucleus from within the egg.
If you look closely at the tip of the needle, you can just make out the genetic
material being drawn out.
|
| |
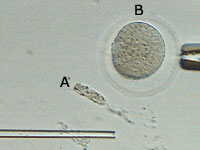
We have released the egg cell's nucleus (A) outside of the egg. This nuclear
material will no longer be needed. What remains is an "enucleated" egg (B) that
still contains protein, RNA molecules, and other important factors that will
ultimately help to establish embryonic stem cells.
|
| |
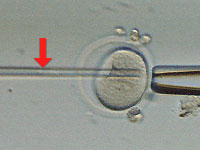
Next, we inject the nucleus (at arrow) from a donor cell into the enucleated
egg cell. In the future, such a donor cell might be a skin cell from a disease
sufferer whom doctors hope to treat using the patient's own stem cells grown in
culture; the procedure would be essentially the same as we're showing here.
Once again we ease the tip of the glass needle through the zona pellucida and
deep into the enucleated egg cell, where we then deposit the donor nucleus.
|
| |
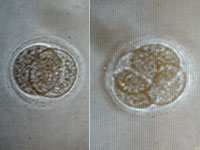
After we complete the nuclear transfer, we "activate" the unfertilized egg cell
using a chemical or electrical treatment that stimulates cellular division. The
first division results in two cells (left image), the next makes four cells,
and so on. This structure is now termed an embryo.
|
| |
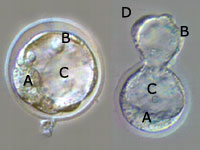
Three and a half days after division begins (in mouse embryos), the
proliferating cells form a structure called a blastocyst. It is 100 to 150
micrometers wide, or roughly the same size as the egg cell. The blastocyst has
only three parts: the inner cell mass (A), the trophoblast cell layer (B), and
the inner cell cavity (C). The inner cell mass is the part that in nature goes
on to form the embryo after implantation in the womb, and thus it also contains
the embryonic stem cells; the trophoblast layer goes on to form part of the
placenta. The righthand image shows the blastocyst "hatching" (D) from the
protective zona pellucida, which it has to do in nature to implant in the
uterus.
|
| |
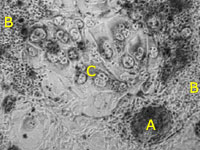
By placing the hatched blastocyst onto a tissue culturing dish, we can
encourage several different types of cells to grow: colonies of embryonic stem
cells from the inner cell mass (A), membrane-like cells (B), and placental
cells (C). Only the embryonic stem cells are able to continue growing under
these conditions, and over time they will multiply to the point that we can
expand the culture to more dishes, a process called "passaging."
|
| |
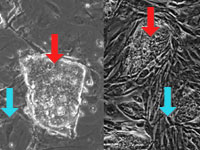
At left are images taken from two different embryonic stem cell (ESC)
cultures—see red arrows—one from mouse (left) and the other from
human (right). Both mouse and human ESCs form compact colonies containing
hundreds of individual cells that look incredibly similar even to the trained
eye. Also shown are "feeder cells" (blue arrows). Typically these are mouse
connective-tissue cells, which supply important nutrients and hormones to the
growing ESC cultures. Scientists are investigating ways to maintain ESC
cultures without feeder cells.
|
| |
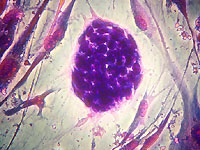
In the center of this image is a single human embryonic stem cell colony that
has been stained to highlight both the individual cells within the colony as
well as the surrounding feeder cells. This small embryonic stem cell colony
contains approximately 50 to 80 individual cells, each measuring about 10
micrometers (or 0.000010 meters) wide.
|
| |
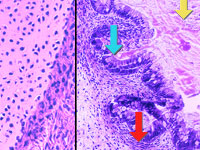
Researchers can use embryonic stem cells (ESCs) to study the development of
specific cells and tissues. Shown here are two types of tissue that we've
grown from federally approved human ESCs. On the left is a section of cartilage, whose cells (in
purple) have secreted a large deposit of collagen (in pink). On the right is a
complex section of intestine or gut tissue. Gut cells (blue arrow) have
secreted mucous-like material into a central cavity (yellow arrow). The ESCs
have also formed muscle (red arrow). In the future, such specialized cells and
tissues grown from ESCs generated by nuclear transfer may help treat disease, hence the term "therapeutic
cloning."

|
|
|
|
|
|
|


