|
Carbon. Oxygen. Iron. Many of us may think of the various chemical elements
as, well, elemental—created by nature, stable and unchanging. But
scientists have now synthesized nearly two dozen novel elements, most of which
are highly radioactive and break apart in a fraction of a blink of an eye. In
the following quiz, test your knowledge of some chemistry basics and learn more
about scientists' quest to produce a more stable superheavy element in a realm
they call the "Island of Stability."—Susan K. Lewis
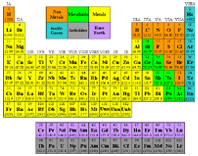
The periodic table is a chart of all known chemical elements, both
natural and synthesized. Why are the elements arranged in a curious pattern of
unequal rows and columns?
- to reflect chemical properties
- to indicate when they were discovered
- to leave room for notes
Hydrogen is the first element on the table, helium the second, lithium
the third, and so on. What determines an element's numerical order?
- its atomic weight
- the number of protons it has
- when it was discovered
Most elements were created inside of stars, but scientists have now made more than 20 elements in laboratories. What was the first element synthesized in a lab?
- technetium (element number 43) in 1937
- neptunium (element number 93) in 1940
- nobelium (element number 102) in 1958
Who officially names a newly discovered element?
- the researcher(s) who discovered it
- the institution where it was discovered
- an international group of chemists
What everyday object makes use of a synthesized heavy element?
- a smoke detector
- a microwave oven
- a fluorescent light
In 1952, elements 99 and 100 were discovered. Where were they
found?
- in a particle accelerator in Stockholm
- in pitchblende, a uranium-rich ore
- in debris from a hydrogen bomb test
The nuclei of elements with a so-called "magic number" of protons tend
to be more stable. What is the heaviest element found in nature with such a
magic number?
- calcium
- lead
- nickel
In the 1960s, physicists predicted that if element 114 could be made, it would be more stable than other superheavy elements. In 1998, when scientists finally created a single atom of element 114, it survived for how long?
- 30 seconds
- 30 hours
- 30 days
Answers
|