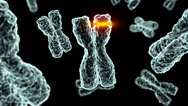
Cracking Your Genetic Code
We are on the brink of a new era of personalized, gene-based medicine. Are we ready for it? Airing March 28, 2012 at 9 pm on PBS Aired March 28, 2012 on PBS

Program Description
(Program not available for streaming.) What will it mean when most of us can afford to have the information in our DNA—all six billion chemical letters of it—read, stored and available for analysis? "Cracking Your Genetic Code" reveals that we stand on the verge of such a revolution. Meet a cancer patient who appears to have cheated death and a cystic fibrosis sufferer breathing easily because scientists have been able to pinpoint and neutralize the genetic abnormalities underlying their conditions. But what are the moral dilemmas raised by this new technology? Will it help or hurt us to know the diseases that may lie in our future? What if such information falls into the hands of insurance companies, employers or prospective mates? One thing is for certain: the new era of personalized, gene-based medicine is relevant to everyone, and soon you will be choosing whether to join the ranks of the DNA generation.
The program was produced in association with The Hastings Center.
Transcript
CRACKING YOUR GENETIC CODE
PBS Airdate: March 28, 2012
NARRATOR: This is no ordinary flash drive. From a small company called Knome, it contains a complete digital record of a person's genetic code, all six billion letters of it.
NATHANIEL PEARSON (Knome, Inc.): Your D.N.A. is what makes you unique. It governed how you grew in the womb and how you look today. And, until now, only a few hundred people in the world have had a chance to see their whole genome and try to understand it.
NARRATOR: Few could afford the cost: $350,000, just three years ago, but that's changing.
FRANCIS COLLINS (National Institutes of Health): It's almost amazing to be able to say that each of us will have the chance to have our complete genome sequenced, for less than $1,000, in the next four or five years, but it's true.
NARRATOR: The result could be a revolution in medicine: using genetic information to diagnose and cure disease.
JOE BEERY (Noah and Alexis Beery's Father): If you go back and you look at some of the home movies that we took, and you see Alexis falling down, and you look at her now, you think, it's unimaginable that she was actually that same child. I really do believe that whole-genome sequencing really, really saved Alexis' life.
NARRATOR: But it could also lead to wholesale invasions of privacy and an ethical quagmire.
JAY ADELSON (23andMe Client): There's a lot of fear about, say, insurance companies or other professionals being able to access that data.
RUDOLPH TANZI (Massachusetts General Hospital): And then the company geneticist says, "He has an increased risk for cancer, okay? Just don't interview him; he'll never know." Do you want that? Because that is potential reality.
NARRATOR: Thousands of years ago, the Ancient Greeks were given some famous advice: "Know thyself." Today, when those words are a biotech company motto, they present a new kind of challenge. Just how well do you want to know yourself in the age of personal genomics?
Up next on NOVA: Cracking Your Genetic Code.
A few years from now, you may boot up your tablet to find a life-changing report: a report on your own, personal genetic code, on the thousands of genes that spell out your body's instructions. Deciphered, your genes will reveal your risks for one disease after another, those you may get yourself and may pass on to your children.
How will it feel to have this information? You may find out sooner than you think.
GREGORY STOCK (Author, Biophysicist): We're entering an era of unprecedented self-knowledge. We are really beginning to come to understand the living processes that constitute ourselves, where we can begin to intervene to take control of our own future.
FRANCIS COLLINS: Genomics offers us the chance to look, in the most precise way, at what the causes of illness are and how to prevent and treat illnesses with that information. And we have that opportunity, now, in front of us.
NARRATOR: This could be your future:a new kind of personalized medicine based on your genetic code, one that predicts risks, so you can stop diseases before they appear, if there's a way of stopping them.
RUDI TANZI: But what if you can't? What if you have a gene mutation that says, doesn't matter how you live your life, doesn't matter what drugs you take, you will get this disease and probably before 50 years old.
CATHERINE ELTON (Journalist): Not everybody can handle genetic testing. And this information affects the way you live the rest of your life, if you are going to get a disease.
NARRATOR: But while some sound notes of caution, the science is rushing ahead and is now taking on medical challenges once thought impossible.
Consider Andrew Schmitz, a bubbly five-year-old, who has no idea his life hangs in the balance.
PAULA SCHMITZ (Mother of Andrew Schmitz): It started with high fevers and joint pains. And then, July he had his first stroke. And then he had two in October and one in November that required brain surgery. And then, his last one, number five, was a week ago.
NARRATOR: Andrew is at the center of a medical mystery. His parents have consulted dozens of specialists, but so far his symptoms defy diagnosis. He gets steroids to calm his immune system and has been on and off chemotherapy. Nothing seems to work.
At Children's Hospital, in Milwaukee, Andrew's pediatrician, Dr. Sheetal Vora, assesses his condition and the toll being taken by the drugs used to treat him.
SHEETAL VORA (Children's Hospital of Wisconsin): It pains you, because I have been there with this family from the beginning, and I have seen the ups and downs and told them the brutal truth, that you use all these medications, but they also can have their side effects.
NARRATOR: Desperate for a diagnosis, Dr. Vora has brought in geneticist Howard Jacob.
HOWARD JACOB (Medical College of Wisconsin): Right now, we don't know what is the cause of his disease. It's possible that it's environmental, or it's possible that he had some type of an infection. In general, though, somebody else should have it. Why doesn't anybody else have it in the family? What about in the community? So, a more plausible explanation is that it's genetic.
So if it happens to fall within a gene…
NARRATOR: If Jacob is right, there's a chance that Andrew's condition could finally be diagnosed, opening up the possibility of a cure.
HOWARD JACOB: So we will do everything we can to comb through his genome and see what we can find.
NARRATOR: To fulfill this promise, Jacob will be putting Andrew in a select group, those who have had their genomes, that is, all the genetic material contained within their cells, read out, letter by chemical letter, six billion, in all.
To start the process, a nurse draws Andrew's blood. The next day, it arrives at Illumina, one of a handful of companies that reads, or sequences, genomes.
In the lab, the blood is processed to extract its genetic material. As proteins and fats are washed away, delicate fibers clump together. This is D.N.A., life's master molecule. Next, the D.N.A. is sheared into fragments, making it easier to sequence.
It is such a complex task that sequencing the first human genome took 13 years, three billion dollars and hundreds of scientists. When the first draft was finished, in 2000, it was hailed as one of humanity's great achievements.
ERIC LANDER (Broad Institute of M.I.T. and Harvard): This is all the instructions there are, telling you all the tricks cells use to actually go from being a single cell to a whole grown up individual. All those recipes are written in exactly the same language.
NARRATOR: A language whose alphabet consists of four chemicals:each known by its initial:A, T, C and G. Strings of these chemical letters spell out some 20,000 genes, on 23 pairs of chromosomes. Genes code for proteins, molecules that do most of the work in our cells and help build parts of our body, from muscles to hair.
And, in the world of genes and proteins, spelling counts. If D.N.A. is copied incorrectly or damaged, spelling errors, known as variants or mutations, crop up.
NATHANIEL PEARSON: Now, when you change the spelling of a gene, sometimes it drastically changes the way that a protein functions, and those are the kinds of changes in the genome that we really look to when we are trying to trace disease, when we are trying figure out what spelling variant in the genome explains, for example, why this child is sick.
NARRATOR: And that's what Howard Jacob will search for. Convinced that a misspelled gene underlies Andrew's condition, he will comb through the boy's genome to find it. But even if he does, it's still a gamble.
HOWARD JACOB: The chances are pretty high that we're going to find something that there's nothing we can do about it. And that's where, I think, a lot of times, people worry about, well, if you can't change it, why do it? And we believe that providing an answer to the family does have merit to the family, even if we can't help his outcome. And so, here, we've decided that it's better to go look and potentially fail by looking than to not have looked and missed an opportunity to succeed.
NARRATOR: As advances in technology drive down the cost of D.N.A. sequencing, it's becoming accessible, not just to the sick, but the curious. And a handful of companies have arisen to meet the demand. They don't offer whole-genome sequencing yet, more of an economy-class genome scan.
One of the best known is the Silicon Valley start-up, 23andMe, co-founded by Anne Wojcicki.
ANNE WOJCICKI (23andMe): From day one, when we started this company, our goal has been, "How do we make genetics accessible?" And we started off with the genotyping technology, because it's a fabulously robust technology. It's incredibly reliable, and, most importantly, it's inexpensive.
NARRATOR: Human genomes are 99.9 percent identical, but by analyzing the D.N.A. in your spit, 23andMe will show you a million sites in your genes where the spelling sometimes differs between people. These one-letter variants may predispose you to certain traits and diseases.
A million letters may sound impressive, but that's far less than one percent of your D.N.A.
JONATHAN ROTHBERG (Inventor of Next Generation Sequencing): Genotyping is not D.N.A. sequencing. Genotyping is identical to looking at 100 words in a 600-page novel and believing you know everything about Tolstoy.
NARRATOR: Genotyping can explain odd traits, such as why some people find Brussels sprouts bitter, but when Jay Adelson wanted to know his chances of getting the brain disorder known as Parkinson's disease, he found the results far less clear.
JAY ADELSON (23AandMe Client): There is something called an odds calculator. And that odds calculator says I have roughly a 60 percent chance of contracting Parkinson's disease. And my father has it, but his parents and his grandparents…no one had Parkinson's disease. So, while it's a genetic trait that passes down, it doesn't necessarily mean that I'm going to contract it. And so, I spent a lot of time trying to understand what this meant.
TOM MURRAY (The Hastings Center): Almost all the evidence we're going to get will be, not hard, determining facts about our futures, but it'll be probabilistic information. It will tilt the odds one way or another.
ROBERT GREEN (Brigham and Women's Hospital): And we're not very well prepared, as a society, to, sort of, negotiate those risk elements. And some people will over-interpret those risks, and they will rush out and try to get diagnostic tests, or they'll try to get surveillance test to try to help them interpret what this information means or doesn't mean.
NARRATOR: Given such concerns, critics argue that genetic information should only come from a medical professional. Anne Wojcicki disagrees.
ANNE WOJCICKI: As of today, we're the only company that allows you to go direct, to the Web site and not require you to go through a physician. And, again, I think this is really core to our belief that this is your genetic information and something that's fundamentally about you and that you should have the right to get access to that information.
NARRATOR: Even the head of the National Institutes of Health, Francis Collins, decided to take the genomic plunge. He submitted his D.N.A. to three genotyping companies, including 23andMe.
FRANCIS COLLINS: I was a little on the cynical side about, "Yeah, yeah, these are early days, and how do I know they even got the results right?" But opening up that Web site, and beginning to look down the list of things where I turned out to be at higher than average risk got my attention.
NARRATOR: All three companies agreed Collins was at a substantially increased risk for getting type 2 diabetes.
FRANCIS COLLINS: And it got me motivated. I am 27 pounds lighter today than I was two years ago, and I'm working out three times a week. And the chance to have a little bit of a prediction about your future, as imperfect is it is right now—and it's very imperfect—could still be a teachable moment.
NARRATOR: But some of the results were contradictory.
FRANCIS COLLINS: Prostate cancer was the most glaring example. One company said higher than average risk; one said about average; the other said lower than average risk.
NARRATOR: So what's going on? According to neurogeneticist Rudy Tanzi, companies often look at different parts of genes and make predictions based on incomplete data.
RUDY TANZI: And even when we have that full set of genes where these variants increase your risk and these variants protect you, knowing how they work together or independently to come up with a real number? Good luck.
And remember, most of those variants are going to work together with your lifestyle. They're not guaranteeing anything. It depends on how you eat, do your exercise.
DAVID ALTSHULER (Broad Institute of M.I.T. and Harvard): I always wonder, is it possible that the person that is told they are not at high risk will do less and maybe be harmed?
RONALD GREEN (Dartmouth College): Oh, I don't have a cardiac problem, genetically. Now I'm going to go out and eat rich foods night and day, and so on. So, information is always hard to handle.
NARRATOR: One example is a piece of genetic information so potentially disturbing that even a founder of modern gene science refused to take a chance on running into it.
KEVIN DAVIES(Author, $1,000 Genome): James Watson, the man who co-discovered the double helix of D.N.A., one of the very first people in the world to have his genome completely sequenced, even before that was done, he said to the scientists who were doing the sequencing, "There's one gene I don't wish to know anything about."
NARRATOR: Known as ApoE4, on chromosome 19, it has been associated with late onset Alzheimer's, the leading cause of dementia in the elderly. The variant increases one's risk three- to tenfold.
RUDI TANZI: But what does that mean? What does it mean to say, "You have a tenfold-increase risk over someone who doesn't have it?" Can you then covert that into an absolute lifetime risk type of number to say, "Here's your percent chance of getting the disease?" No. Because you need to know what other genes work with ApoE. You need to understand how lifestyle is working together with ApoE.
NARRATOR: In fact, many people with ApoE4 never get Alzheimer's, while others with the disease don't have the variant.
ROBERT GREEN: I think the real key here is to disabuse people of the misunderstanding that genetics is wholly deterministic. In a few rare instances, there is a tight linkage between a particular mutation and a disease, but the vast majority of genetic information is largely probabilistic.
NARRATOR: Yet, some genes speak louder than others. Although we get two copies of most genes, one from each parent, certain dominant genes confer a trait of their own. And certain dominant disease genes, although rare, will eventually make you sick.
As a volunteer for the Huntington's Disease Society, Katie Moser works with people in this rare category, among them Meghan Sullivan.
Four years ago, Meghan was a high school student with everything to look forward to, but, with college, came a set of heartbreaking symptoms.
MEGHAN SULLIVAN (Huntington's Disease Patient): I was a perfectionist, but all my grades, my sophomore year, were Ds. I had no idea why, because, in high school, since 5th grade, I made the honor roll every single time.
NARRATOR: The reason for Meghan's plummeting grades, sudden movements and stumbling speech is a snippet of genetic code that repeats many times instead of a few, causing Huntington's disease. The mutation creates an elongated protein in the brain, which is as toxic to Meghan as it was to Katie's grandfather, who also had Huntington's.
And if he passed this gene to Katie's mother, Katie had a 50 percent chance of carrying it herself. For years, she wondered if she should get tested.
RUDY TANZI: In the case of a gene mutation that guarantees the disease, that's a real tough decision, because there, there's no hope. If you get the wrong answer, it's somewhat of a death sentence.
KATIE MOSER (Huntington's Disease Society): I decided to get tested for the gene, because I wanted to be able to plan my life financially, physically, emotionally. I wanted to know if someday I would start showing symptoms, but my family did not want to know.
NARRATOR: Tests confirmed that Katie will eventually get Huntington's disease, but knowing has had repercussions:dates who disappear; relatives who won't speak to her, since they must now confront their own genetic status.
"The burden of knowing" is what journalist Catherine Elton calls it.
CATHERINE ELTON: In this age of personal genetic testing, it's not personal. People exist in families, and by the nature that you have tested, you are revealing this information to people who may not want to know.
NARRATOR: Elton, herself, was offered a test for mutations in BRCA1 gene, linked with high chance of getting breast or ovarian cancer. Her mother, her grandmother and her aunt had their lives cut short by these diseases. If Elton had the mutation, she could minimize her risks or more by having her breasts and ovaries removed. If she wanted children, she would have to have them before surgery.
CATHERINE ELTON: I didn't want those results. I didn't want to have that in the back of my mind and maybe make me settle for the wrong guy and rush into having kids before I was ready. And I think there is a real fine line between avoiding death and ruining your life.
NARRATOR: But Elton, too, has paid for her decision. In 2008, while pregnant with her second child, she was diagnosed with breast cancer. Yet, despite the ordeal of surgery and chemotherapy, and the risk the cancer might come back, she's convinced she did the right thing by not getting tested in her 20s.
CATHERINE ELTON: If I had made those decisions at 27, I can't even believe what I would have missed out on. And as the technology becomes more accessible, people just think, "Well, this is what you do." But I happen to believe that some of the costs of knowing our genetic destiny can outweigh some of the benefits.
NARRATOR: While Catherine Elton sees genetic information as potentially damaging, others see her disease-causing variant, BRCA1, as one of the first you can do something about, a so-called "actionable" gene.
LEROY HOOD (Institute for Systems Biology): These are genes where, if you know the patient has a variant in that gene, of a particular type, you can actually counsel them on things that will improve their wellness.
NARRATOR: One of these variants causes deadly blood clots, but needn't, if you avoid long periods of immobility or take blood thinners. Another variant tells us we could fall victim, even in our teens, to a heart attack.
NATHANIEL PEARSON: As a healthy adult, if you learned about one of those variants and found out you carried it, how would that change your life? Well, you might actually invest in defibrillator machines for your home or your workplace. You might actually change your vacation plans, in terms of whether you want to do really strenuous sports activities or shock yourself by jumping into cold water.
NARRATOR: So far, scientists have found about 200 actionable genes, including one that boosts your chance of colon cancer by age 45.
LEROY HOOD: So, what can you do about that? Well, if you start colonoscopies at 25 or so, you can actually keep people free of the disease.
RUDY TANZI: If you look at a personalized-medicine approach, the mantra is "early prediction, early detection." You want to know pre-symptomatically if you're in trouble, so that you can start treatment to nip the disease in the bud stage, prevent it before it strikes.
FRANCIS COLLINS: And if you do get sick, and your doctor has to make a decision about how to treat you, there are going to be signals in your instruction book to say, "Not that drug. Use this one instead."
NARRATOR: Of course personalized medicine only works if we know the gene variant responsible for a condition.
Back at Milwaukee's Children's Hospital, the search for the genetic cause of Andrew's illness is beginning. His decoded genome has arrived from Illumina. Sequencing took 45 days and cost $7,500. The next challenge, and the major expense, is figuring out what it all means.
To find Andrew's variants, Jacob's team will compare his genome with thousands of others, but mainly with the reference genome sequenced by the Human Genome Project.
DAVID DIMMOCK (Medical College of Wisconsin and Children's Hospital of Wisconsin): So this, here, is the reference sequence.
HOWARD JACOB: And so the computer's the first pass, it basically goes through and asks a question at each point across Andrew's D.N.A.:"Are you the same or different from the reference?" And when we see a difference, we then ask a question:"Is that difference meaningful?"
NARRATOR: Meaningful in that the variant must be unique to Andrew, and a possible cause of disease. But as the list of suspects shrinks from three million to a few thousand, success proves elusive.
Meanwhile Paula and Mike Schmitz keep Andrew's life as normal as possible.
MIKE SCHMITZ (Father of Andrew Schmitz): We had hoped that he would progress a little better with his rehab, with his walking. And it's hard to live every day with the anxiety of not knowing what's next, you know? And with Andrew, there's always something next. But, there is hope.
NARRATOR: One source of this hope is another Wisconsin boy. From age two, Nicholas Volker struggled with a condition that ate holes in his intestines. When treatment after treatment failed, Nick's doctors turned to Howard Jacob and gene testing.
HOWARD JACOB: That's for your birthday. Shall we open it?
NARRATOR: Now, nearly two years later, Nick is paying Jacob a visit.
HOWARD JACOB: Ask the question, "How many Ph.D.s does it take to assemble a Green Lantern?"
NARRATOR: Nick is the model for the success of this approach. His illness was traced to a gene on chromosome X, called XIAP, linked with immune disorders. A single letter was out of place:a G that had mutated into an A.
NICHOLAS VOLKER (Patient of Howard Jacob): I want to do the tank.
HOWARD JACOB: You want to do the tank?
NICHOLAS VOLKER: Yeah.
HOWARD JACOB: I was afraid you were going to say that.
We found one single letter change in this gene, XIAP, which now was unique. Nobody else has the same variation that Nick has. And that's meaningful, because that means that letter is so important, that anybody who would have had this particular variation would die.
NARRATOR: This single variant caused Nick's symptoms, but a transplant, giving him a donor's immune system, without the misspelling, appears to have saved him.
In Boston, genomics is also being used in the battle against better known conditions, like cystic fibrosis, or C.F.
Michael McCarrick, age 29, knows its ravages first hand.
MICHAEL MCCARRICK (Cystic Fibrosis Patient): How I've experienced my decline has been sort of…in the beginning of my life, I played a lot of sports, and I had fun playing a lot of sports, and then, suddenly, I couldn't run, but I could walk long distances, and now it is like walking, itself, is difficult.
NARRATOR: Like many C.F. patients his age, Michael is facing end-stage lung disease.
AHMET ULUER (Children's Hospital Boston): Where Michael is right now and what he is dealing with is the torture we don't like to watch our patients go through, the struggle for that next breath.
NARRATOR: Michael may need a lung transplant, but Dr. Uluer hopes a new, gene-based drug will save him instead. Called Kalydeco, it targets a mutation found in four percent of C.F. patients.
MICHAEL MCCARRICK: Every drug can have a side effect. But I am hoping this one is free and clear, because who needs an extra problem? So, hopefully it's a magic bullet.
NARRATOR: The development of a drug for cystic fibrosis is especially gratifying to Francis Collins. Decades ago, Collins and a team of scientists, using rudimentary technology, set out to find the genetic defect behind the disease.
FRANCIS COLLINS: Back in the 1980s, this was like looking for a needle in the haystack, in the dark, with thick gloves on. And one day, in the spring of 1989, the data came across showing that individuals with cystic fibrosis were missing just three letters of that code.
NARRATOR: Just three letters, that is, from each copy of a gene called CFTR on chromosome 7. Because the mutation is recessive, only those who inherit a copy from both parents get the disease.
While we now know 1,800 different mutations in this gene can cause C.F., Collin's team found the main one, truly his needle in the haystack.
FRANCIS COLLINS: When this was announced, in August of 1989, the excitement was palpable. And I think many of us thought maybe this is the launch of a therapeutic effort that could happen pretty quickly.
NARRATOR: In reality, a clinical breakthrough would take another 20 years of research and hundreds of millions of dollars.
Some of the most successful work has been done at Vertex Pharmaceuticals, in San Diego, where scientists are trying to fix the defective protein made by the C.F. gene.
The normal protein creates an opening for salts to move across cell membranes, keeping them moist enough for tiny hairs called cilia to beat and remove mucus.
FREDRICK VAN GOOR (Vertex Pharmaceuticals Incorporated): In C.F., the cilia are not able to clear out the bacteria and the mucus, and this leads to chronic infection. So the first challenge was to find molecules that help the protein work better.
NARRATOR: Drawing on a vast chemical library, the research team employed a small army of robots to test 600,000 compounds on cells taken from C.F. patients. To date, two drugs have shown the most promise; one of them:Kalydeco. When tested on C.F. cells, it helped the protein function, allowing salts and fluids to flow across membranes. Van Goor could watch the result:cilia beating, clearing away mucus, while untreated cells languished.
FREDRICK VAN GOOR: It was an exciting moment, where we really felt we are on the right track, designing drugs to fix a specific problem in a protein caused by a mutation.
NARRATOR: Whether Kalydeco will work for Michael McCarrick, whose lungs are severely damaged, remains unclear. But younger patients, like Paul Glynn, are seeing a world of difference.
PAUL GLYNN (Cystic Fibrosis Patient): It's a lot easier, 'cause I am not coughing, and I can breathe easier. And then my weight, it's been going up.
NARRATOR: Since taking the drug, Paul has gained 12 pounds, enough to make the local football team. For him, the hope is that C.F. will become a manageable disease, and regular hospital visits a thing of the past, which may even make the drug cost-effective, despite its price tag:as much as $294,000 year.
In Boston, meanwhile, at Massachusetts General Hospital, doctors are using new gene-based drugs to target the most common disease of the genome: cancer.
Tom Garpestad is a 50-year-old building contractor. He was stunned to learn he had the skin cancer called melanoma.
TOM GARPESTAD (Melanoma Patient): Melanoma is the cancer that doesn't act like cancer. I had no symptoms, no weight loss, no night sweats. I felt perfectly fine, up until the point that my neck started bothering me.
NARRATOR: Scans revealed that the cancer had spread from his skin to his neck, lungs and liver.
KEITH FLAHERTY (Massachusetts General Hospital): Patients who have melanoma that has spread to other parts of the body from the primary skin site, have, from the time of initial diagnosis, typically a year or less. Tom had already had metastatic melanoma for sufficiently long that, as he walked in the door to our clinic, he was down to a month or two.
TOM GARPESTAD: I talked to my brother, who is a doctor, and he started telling me how severe melanoma is. You know, the average life expectancy of somebody with metastatic melanoma is nine months.
TODD GOLUB (Broad Institute of M.I.T. and Harvard): Research labs weren't even working on it that hard, because it seemed to be completely intractable; nothing would ever work. And this all changed, almost overnight, with the sequencing of the melanoma genome.
ERIC LANDER: The idea of sequencing a cancer…it's as big as the human genome. Each cancer cell has an entire human genome in it, just mutated in various ways. And genomics told us all that there was a mutation in many melanomas, in a specific gene that goes by the funny name, BRAF. And it led to the idea that if you could inhibit this BRAF, you might be able to stop the melanomas.
NARRATOR: Luckily for Tom, gene sequencing revealed he had the BRAF mutation. He could now join a clinical trial of a new drug designed to neutralize its effects.
TOM GARPESTAD: The cancer was getting very aggressive. And then they started me on the BRAF medicine, and, within a week, I could feel the tumors shrinking.
NARRATOR: The BRAF mutation results in a defective protein that signals cells to divide uncontrollably. The drug binds to this protein, stopping both the signal and the cancer. Unlike chemotherapy drugs, this one kills only cancer cells, nothing else.
PET scans of patients reveal tumors that once riddled bodies, shrinking or vanishing within weeks.
KEITH FLAHERTY: We had a situation in a disease that was never responsive to therapy 90-plus percent of the time. To have that turn around and have it be 90-plus percent of the time that the treatment worked, that's when we knew we had completely crossed into uncharted territory.
NARRATOR: Only two months after lying, near death, in the hospital, Tom was back to his old life.
TOM GARPESTAD: People close to me, seeing me back on the job, just getting out there and doing things again? It was amazing. I was always thinking, "Hey, how sick was I two months ago? Look at me now."
NARRATOR: But that doesn't mean the war is over. To Keith Flaherty's dismay, scans show melanoma returning in many patients. Cancer cells, like viruses and bacteria, can evolve to resist drugs, even those that target genes. But now, the ability to compare cancer genomes before and after treatment is allowing scientists to see how resistance develops.
ERIC LANDER: The goal is to use the cancer genome itself to tell us how to defeat cancer, tell us what's wrong in a cancer and where we should hit it, tell us how it's becoming resistant and how we can block it.
NARRATOR: With resistance, new mutations arise in melanoma tumors, and once again defective proteins ignite the cancer.
So, now, Tom is taking a second drug, which targets a mutation linked to these relapses.
Eight months after starting his treatment, he awaits the results of a new set of scans.
DONALD LAWRENCE (Massachusetts General Hospital): The scans do show some re-growth, but only in a limited area. In all the other areas where we have seen signs before, things look great.
NARRATOR: Afterwards, Tom takes in the news.
TOM GARPESTAD: I probably wouldn't be alive right now, you know? So, I mean, I'll take, I'll take the eight months. You know, I mean, it's still a miracle drug, I think.
NARRATOR: At present, patients like Tom are getting between two and 18 extra months of quality life from the new treatment. But genomics is also helping those for whom there are no targeted drugs.
Thousands of breast cancer patients are taking a gene test, which tells them how aggressive their cancer is, and whether they need chemotherapy or can safely skip it.
The hope is by studying cancer genes, we'll come up with a cocktail of drugs like those used to treat tuberculosis or H.I.V., to cure cancers or keep them in check.
TODD GOLUB: The big difference, though, is that now, really for the first time, we can think about the right combinations of drugs, based on the genome, based on the science of what's going on inside the cancer cell.
ERIC LANDER: This is not a project for my life; this is a project for my kids; this is a project, ultimately, for their kids. But if, in the course of this century, we have a complete roadmap of what a cancer knows how to do, that will be a mind-boggling advance in medicine.
NARRATOR: Another extraordinary advance would be to eliminate inherited diseases before birth.
We're already taking the first steps:by fertilizing an egg and producing an eight-cell embryo, you can pluck off one of those cells and analyze its genes. Then you can screen that embryo for a host of diseases, using a technique called "preimplantation genetic diagnosis," or P.D.G.
MARK HUGHES (Genesis Genetics Institute): When we first started performing this technology, I think our biggest worry was, "Aren't we going to create some kind of a birth defect that is maybe even worse than the disease we're trying to avoid?" And what we learned was that the embryo doesn't seem to mind.
NARRATOR: At the Genesis Genetics Institute, Mark Hughes and his team are using P.G.D. to test embryos for mutations that can give rise to over 300 diseases, including Huntington's and cystic fibrosis.
Only embryos free of certain mutations are implanted in the mother.
MARK HUGHES: So this is the mutant gene, and we have the normal gene over here.
Tens of thousands of healthy babies have been born to couples who otherwise would have been afraid to have a child, because the disease that they were at risk of giving their child was so severe.
NARRATOR: P.G.D. can also be used to test for traits like gender, leading ethicists to ask if designer babies are in our future.
RONALD GREEN (Dartmouth College): I think we are going to see people using genetics to select traits. Some of it will be relatively benign:"I want a child with this hair color or eye color."
GREGORY STOCK: As to where our ability to really intervene in our own living processes is really going to lead us, we simply do not know. And that is the promise and that is the threat of this period.
NARRATOR: Some fear P.G.D. could even lead to a new kind of eugenics and the sort of genetic elite depicted in the movie GATTACA.
DOCTOR (GATTACA Film Clip): Your extracted eggs, Marie, have been fertilized with Antonio's sperm. After screening we are left with two healthy boys and two very healthy girls. I have taken the liberty to eradicate any prejudicial conditions:premature baldness, myopia, alcoholism and addictive susceptibility, propensity for violence, obesity, etc.
NARRATOR: Fortunately, the traits a genetic elite would want—intelligence, physical ability, even height—are so complex, scientists assure us we won't be selecting them in embryos anytime soon.
ERIC LANDER: Trying to predict height, well, we already know there's at least 180 genes involved in height, and each contributes a little bit. It isn't going to be easy to go take some embryo and sort out which of these 180 are in which of these forms. You really, really, really want to have a tall person, go marry a tall spouse. It's just more efficient.
NARRATOR: Even so, GATTACA raises real concerns.
RONALD GREEN: One of the negative implications of this new genetic knowledge is that we're going to start thinking of ourselves more in genetic terms than we ever have before. Do I want to date that individual? What's her genetics? There's always been a tendency to engage in deterministic genetics, and I think scientists and medical people and educators must make very clear the limits of that point of view.
PATRICIA WILLIAMS (Columbia University): We are deeply affected by the kind of food we eat, the air we breathe, by the kind of good luck or bad luck that shapes our lives, like education and money, and by the real romance of simply falling in love with an unlikely partner. We narrow our vision if we focus or fetishize upon genetics.
NARRATOR: But as the cost of a sequenced genome falls, new generations may not get far into the world without one.
JONATHAN ROTHBERG: And I envision a day when every child is born, they prick that child's heel, and that D.N.A. from that child is decoded right at birth.
NARRATOR: For 14-year-old twins Noah and Alexis Beery, sequencing at birth could have made a world of difference. Today, it's hard to imagine that just two years ago, Alexis was fighting for her life.
ALEXIS BEERY: The only really vivid memory that I have of that is just…simple as just seeing red, blue and white lights just flashing everywhere. That's definitely one of my, like, number one, kind of like, nightmares that I still have.
JOE BEERY: After Alexis and Noah were born, they didn't reach any of their milestones. They didn't crawl on time, they didn't walk on time. And we knew, pretty early, that something wasn't right.
NARRATOR: The twins were diagnosed with cerebral palsy. Then, at age five, Alexis got worse.
RETTA BEERY (Noah and Alexis Beery's Mother): She started losing more and more ability to walk during the day. She started losing ability to sit up by 10:30, 11:00 in the morning. She could no longer swallow by that time. And so, that's not indicative of cerebral palsy.
NARRATOR: Retta began doing research. One day, she came across a rare condition that mimics cerebral palsy, a condition that could be treated with the brain chemical dopamine. Dopamine is crucial to our bodies' ability to move.
And the morning after Alexis took it, she woke up to a new world.
JOE BEERY: She was walking, she was talking, she was using her arms, she was whistling.
NARRATOR: Noah also responded. Yet the twins still had health issues, especially Alexis.
RETTA BEERY: We actually almost lost her on a couple of occasions. We had paramedics coming into our house, trying to get her breathing, and we were back into that world of unknowns.
NARRATOR: Until, that is, Joe asked his new employer, a biotech company, for help getting the twins' genomes sequenced.
JOE BEERY: We discovered, through sequencing, that there was a second problem associated with a rare mutation that they had.
NARRATOR: The mutation was suppressing yet another brain chemical. Another drug solved that problem, too.
JOE BEERY: Whole-genome sequencing really saved Noah and Alexis's life. Had that happened at birth, we would have 15 years less pain and suffering and probably millions of dollars of cost.
NARRATOR: Yet sequencing at birth may have its downside.
RONALD GREEN: One of my concerns is the testing of children raises many questions, including stigmatization. "Oh, I'm not going to let Mary play soccer, because she's got this cardiac risk." So we do have a problem of invasion of the child's privacy.
NARRATOR: And what about your privacy? What if a company asks to use your D.N.A. for research, promising you'll remain anonymous.
PATRICIA WILLIAMS: Now the question is whether or not that anonymized information is truly anonymized, just because they take your name and your social security number off. At some point in the future, it may be that you don't need a social security number, that you don't need to give your name, because your genetic information will reveal you so precisely that we'll have to develop a whole new definition of what we mean by anonymity.
NARRATOR: And what if your D.N.A. reveals you're at increased risk for an incurable disease, say, Alzheimer's?
TOM MURRAY: Can a company that sells long-term care insurance ask you about that? And can they use that result to either affect your premiums or even deny you insurance, entirely because you're such a bad bet?
NARRATOR: In most states, the law already allows long-term care, disability and life insurers to discriminate based on genetics.
RUDI TANZI: I would not make one single base of my D.N.A. sequence available publicly, in a million years. There's too much risk. You don't know what's going to happen in the future with insurance. And think about your company going down the tubes, and now you have to get a new job. And, yeah, employers can't discriminate, but they happen to find out on your Facebook page, under D.N.A., click:there's the sequence.
NARRATOR: Everywhere you go, you leave a trail of genetic debris:when you cut your hair, enjoy a meal at a restaurant.
A crime that once seemed like science fiction has become possible with cheap, fast D.N.A. sequencing.
RONALD GREEN: It's been called "genomic hacking." That will be frightful. It will be used to impugn people. Somebody saying he should not or she should not be a candidate for the presidency, because she has the gene for depression. If somebody wants access to your genome for personal, romantic purposes, for economic reasons, for political reasons…in the future, if they want it, they'll go after it.
NARRATOR: Yet when illness strikes, privacy may seem a distant concern, and genomics your best hope.
In Milwaukee, after months of searching through Andrew's genome, finally, there's a discovery.
HOWARD JACOB: This is a list of genes that we pulled out. So yesterday, at noon, I got an e-mail from Liz saying, "I found a very interesting gene."
ELIZABETH WORTHEY: He's got two protein-coding….
NARRATOR: Geneticist Elizabeth Worthey has found something unusual in Andrew's genome.
HOWARD JACOB: There's two rare variants, one that's never been seen before, and one that's only been seen once, as far as we know.
The next question, then, is, "Does this gene look like it could explain part of Andrew's clinical features?" And in this case, the answer's yes.
ELIZABETH WORTHEY: Mutations in this gene have been linked with susceptibility to recurrent viral infections, which he had.
HOWARD JACOB: I will tell you that this is where we get both excited as scientists and nervous, then, as scientists, because you think you've found it. There's a big difference between thinking you've found it, to, "We've proved it."
NARRATOR: In fact, the suspect gene is soon ruled out, and the family learns the search must continue.
HOWARD JACOB: So, don't worry, until we tell you that we stopped looking, we haven't stopped looking.
PAULA SCHMITZ: Well, we really appreciate it.
NARRATOR: In a field this new, success and failure continually intermix.
In Michael McCarrick's case, the drug targeting his cystic fibrosis mutation helped, but his lungs were already so damaged, he died waiting for a transplant.
Yet Paul Glynn, who used to spend part of every fall in the hospital, is healthier than ever.
Tom Garpestad is in a kind of limbo, with some tumors shrinking, others growing.
As for the Beery twins, their only regret is not getting sequenced sooner.
FRANCIS COLLINS: We have focused so much of our energies on treating people with disease, often advanced disease, and much less effort on trying to prevent that disease in the first place. If we are going shift towards prevention, your genome sequence may be one of the most critical tools you could imagine.
NARRATOR: How can we balance the risks and benefits of this critical tool? This will soon be a question for all of us, as we take up the ancient challenge, "Know thyself," in the genome age.
Broadcast Credits
CRACKING YOUR GENETIC CODE
- WRITTEN, PRODUCED AND DIRECTED BY
- Sarah Holt
- EXECUTIVE PRODUCER
- Laurie Donnelly
- CO-PRODUCER
- Ethan Herberman
- ASSOCIATE PRODUCER
- Julie Crawford
- SENIOR PROGRAM PRODUCER
- Anne Adams
- For The Hastings Center
- CONSULTING EXECUTIVE EDITOR
- Thomas H. Murray, Ph.D.
- CONSULTING SENIOR PRODUCER AND PROJECT DIRECTOR
- Mary Crowley
- DEPUTY PROJECT DIRECTOR
- Jacob Moses
- PUBLIC AFFAIRS EDITOR
- Susan Gilbert
- EDITED BY
- Sarah Holt
- CAMERA
- Stephen McCarthy
Chip Nusbaum
John Chater
Allan Palmer
Rob Lyall
Stephen Kazmierski
Ken Willinger - SOUND RECORDISTS
- George Shafnacker
Doug Kochenbach
Joe McCartan
David Mendez
Doug Dunderdale
Eric Golden
Len Schmitz
Mike Karas
Bob Freeman
Don Hale - NARRATED BY
- Jamie Effros
- MUSIC
- Christopher Rife
- ANIMATION
- Pixeldust Studios
- ADDITIONAL EDITING
- Bill Anderson
Jean Dunoyer - STILLS ANIMATION
- Dan Nutu
- ASSISTANT EDITOR
- David Eells
- ONLINE EDITOR AND COLORIST
- Michael H. Amundson
- AUDIO MIX
- John Jenkins
- RESEARCH
- Rich Remsberg
- PRODUCTION ASSISTANTS
- Nikki Bramley
Andrew Eckmann
Fred Breeze
Joel Van Haren
Harold Lawrence
Nicholas Nutu
Thomas Dooley
Blake Farber
Phil Reilly
Steve Sherric
Mark Scheffler
Matt Tessner
Anthony Ventura
Mary Wiseman - ARCHIVAL MATERIAL
- Howard Hughes Medical Institute
Vanderbilt University Medical Center
National Institutes of Health
Journal of Clinical Oncology
Los Angeles Times
Metrocorp, Philadelphia Magazine
Tim Llewellyn
Retta Beery
Catherine Elton
Rodney Hicks
Janet & Peter McCarrick
Katherine Moser
Gary Porter
Jonathan Rothberg
Cheryl Sullivan Staveley
Getty Images
Framepool, Inc.
Thought Equity Motion - SPECIAL THANKS
- James Noonan, Ph.D.
Michael Snyder, Ph.D.
The Peter MacCallum Cancer Centre
Jason Callahan
Rodney J. Hicks
Steve Weise, MGH
Reisa Sperling, M.D., MGH
Broad Institute of MIT and Harvard
Harold Burstein, M.D.
Allison Reilly Bombara
Levi Garraway, M.D., Ph.D
Janet and Peter McCarrick
Paul & Judith Glynn
Jobe Waters
Amylynn Santiago Volker
Renwood Restaurant
Tartufo's Restaurant
Deluxe Station Diner
Peter Agre
Rahul Dhanda
Joanne Lynn, M.D.
Richard Payne, M.D.
Anita L. Allen, Ph.D., J.D.
Lori B. Andrews, J.D.
Alice Domurat Dreger, Ph.D.
John H. Evans, Ph.D.
Ruth R. Faden, Ph.D., MPH
Mark S. Frankel, Ph.D.
Vanessa Northington Gamble, M.D., Ph.D.
Eric T. Juengst
Michael J. Sandel, D.Phil
Blair Sadler, J.D.
Kim TallBear
For The Hastings Center
- BIOETHICS RESEARCHERS
- Daniel Callahan, Ph.D.
Nancy Berlinger, Ph.D.
Michael K. Gusmano, Ph.D.
Josephine Johnston, LLB, MBHL
Gregory E. Kaebnick, Ph.D.
Karen J. Maschke, Ph.D.
Thomas H. Murray, Ph.D.
Erik Parens, Ph.D. - RESEARCH ASSISTANTS
- Colleen Farrell
Michael Turton
Cameron Waldman
Ross White
Research and development funded in part by The Greenwall Foundation.
- NOVA SERIES GRAPHICS
- yU + co.
- NOVA THEME MUSIC
- Walter Werzowa
John Luker
Musikvergnuegen, Inc. - ADDITIONAL NOVA THEME MUSIC
- Ray Loring
Rob Morsberger - CLOSED CAPTIONING
- The Caption Center
- MARKETING AND PUBLICITY
- Karen Laverty
- PUBLICITY
- Eileen Campion
Victoria Louie - SENIOR RESEARCHER
- Kate Becker
- NOVA ADMINISTRATOR
- Kristen Sommerhalter
- PRODUCTION COORDINATOR
- Linda Callahan
- PARALEGAL
- Sarah Erlandson
- TALENT RELATIONS
- Scott Kardel, Esq.
Janice Flood - LEGAL COUNSEL
- Susan Rosen
- DIRECTOR OF EDUCATION
- Rachel Connolly
- DIGITAL PROJECTS MANAGER
- Kristine Allington
- DIRECTOR OF NEW MEDIA
- Lauren Aguirre
- ASSOCIATE PRODUCER
- POST PRODUCTION
Patrick Carey - POST PRODUCTION EDITOR
- Rebecca Nieto
- POST PRODUCTION MANAGER
- Nathan Gunner
- COMPLIANCE MANAGER
- Linzy Emery
- BUSINESS MANAGER
- Elizabeth Benjes
- DEVELOPMENT PRODUCERS
- Pamela Rosenstein
David Condon - SENIOR PRODUCER AND PROJECT DIRECTOR
- Lisa Mirowitz
- COORDINATING PRODUCER
- Laurie Cahalane
- SENIOR SCIENCE EDITOR
- Evan Hadingham
- SENIOR SERIES PRODUCER
- Melanie Wallace
- MANAGING DIRECTOR
- Alan Ritsko
- SENIOR EXECUTIVE PRODUCER
- Paula S. Apsell
A NOVA Production by Holt Productions, LLC for WGBH in association with The Hastings Center.
© 2012 WGBH Educational Foundation All rights reserved
Image
- (genetic data background)
- © kentoh/Shutterstock
Participants
- Jay Adelson
- 23andMe, Inc. Client
- David Altshuler
- Massachusetts General Hospital
- Alexis Beery
- Joe Beery
- Retta Beery
- Harold Burstein
- Dana-Farber Cancer Institute
- Francis Collins
- Director, NIH
- Kevin Davies
- Author, The $1,000 Genome
- Catherine Elton
- Journalist
- Keith Flaherty
- Mass General Hospital
- Thomas Garpestad
- Paul Glynn
- Todd Golub
- Broad Institute/Dana-Farber
- Robert Green
- Brigham and Women's/Harvard Med.
- Ronald Green
- Dartmouth College
- Leroy Hood
- Institute for Systems Biology
- Mark Hughes
- Genesis Genetics Institute
- Howard Jacob
- Medical College of Wisconsin
- Eric Lander
- The Broad Institute www.broad.mit.edu/about/bios/bio-lander.html
- Donald Lawrence
- Mass General Hospital
- Michael McCarrick
- Katharine Moser
- Tom Murray
- The Hastings Center
- Nathaniel Pearson
- Knome, Inc.
- Jonathan Rothberg
- Inventor, Next Gen Sequencing
- Michael Schmitz
- Paula Schmitz
- Gregory Stock
- Author, Biophysicist
- Rudolph Tanzi
- Massachusetts General Hospital
- Ahmet Uluer
- Children's Hospital Boston
- Fred Van Goor
- Vertex Pharmaceuticals
- Sheetal Vora
- Children's Hospital of Wisconsin
- Patricia Williams
- Columbia University
- Anne Wojcicki
- Co-founder, 23andMe, Inc.
Sources
Links
The Hastings Center
http://www.thehastingscenter.org/Issues/Default.aspx?v=246
The Hastings Center is a non-partisan research institution dedicated to bioethics and the public interest. Here you'll find information on the latest research, along with forums, blogs, and advice on how to deal with difficult bioethical issues.
Genetic Testing and Screening
http://www.thehastingscenter.org/Publications/BriefingBook/Detail.aspx?id=2176
Do you have questions about genetic testing? This site gives a comprehensive background on many common forms of genetic analysis offered by the medical community, and probes ethical dilemmas from prenatal screening to direct-to-consumer genetic testing.
Personalized Medicine and Genomics
http://www.thehastingscenter.org/Publications/BriefingBook/Detail.aspx?id=2200
This website offers information on the ethical issues surrounding the use of genomics to tailor health care to the individual. It explores the complexities of genetically-based health care, risk assessment, and more.
Biobanking
http://www.thehastingscenter.org/Publications/BriefingBook/Detail.aspx?id=2154
Here you will find information on stored biological specimens (such as blood, saliva, and surgical tissue) and how they can be used in genetic and biomedical research. The site addresses the issues surrounding ownership of biospecimens, disclosing research results, and other ethical debates.
The Cystic Fibrosis Foundation
http://www.cff.org/
The Cystic Fibrosis Foundation is a non-profit organization that funds research towards finding a cure for cystic fibrosis.
Books
The $1,000 Genome: The Revolution in DNA Sequencing and the New Era of Personalized Medicine
By Kevin Davies. Free Press, 2010.
Education and Outreach Resources
Preview | 00:44
Full Program
Full program available for streaming through
Watch Online
Full program available
3/29/12
Related Links
-

Genetic Testing Dilemmas
Would you take a test that could tell you more about your future health?
-

Extract Your Own DNA
Behold your very own DNA in this do-it-yourself science experiment.
-
A Gene for Fish Odor
The discovery of a gene that explains an embarrassing body odor offers a little comfort to those who suffer.
-

Chemotherapy and Breast Cancer
For some breast cancer patients, chemotherapy isn't always necessary.
-

Gamers and Genomics
An online game called Phylo taps the brainpower of thousands of players to solve complex problems in genetics.
-

Personal DNA Testing: Expert Q&A
Harvard geneticist Rudy Tanzi offers caveats about commercial DNA testing and addresses a wide range of questions.
-
Ethics of Manipulating Genes
Philosopher Philip Kitcher discusses the moral and ethical implications of molecular medicine in this interview.
-

Cracking the Code of Life
NOVA chronicles the race to reach one of the greatest milestones in the history of science: decoding the human genome.
-

Nature vs. Nurture Revisited
Which dictates our existence, genes or environment? Kevin Davies, author of Cracking the Genome, offers an update.
-
Gene Switches
Some genes turn other genes on and off. In this slide show, see how powerful these gene switches can be.
-

Public Genomes: Expert Q&A
George Church, founder of the Personal Genome Project, answers questions about it and other issues of DNA testing.
-
Forget Fingerprints
After a U.S. Supreme Court ruling, DNA databases are set to expand. How will the decision affect your privacy?
-
Picturing the Molecules of Life
Over the past 50 years, scientific images of DNA, ribosomes, and RNA have catalyzed our understanding of biology.
-

Journey Into Human DNA
Take an animated journey down into the miniscule world of chromosomes, genes, and finally DNA base pairs.
-

Before Watson and Crick
How did scientists discover that DNA was the blueprint of life? Brenda Maddox explains.
-

Four DNA Tests
Would you want to know more about your DNA? See what four types of genetic testing can reveal.
-
Personal Genome Project
Learn why George Church of Harvard Medical School hopes to recruit 100,000 people and sequence all of their DNA.
-

The Musical Geneticist
In this interview, Harvard geneticist and singer-songwriter Pardis Sabeti discusses her life and work.
Major funding for "Cracking Your Genetic Code" is provided by the NIH.
Additional funding for "Cracking Your Genetic Code" is provided by Millicent Bell, through the Millicent and Eugene Bell Foundation. Research and development funded in part by The Greenwall Foundation.
The project described was supported by Award Number RC1RR028476 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Center for Research Resources or the National Institutes of Health.
National corporate funding for NOVA is provided by Draper. Major funding for NOVA is provided by the David H. Koch Fund for Science, the Corporation for Public Broadcasting, and PBS viewers.