Stem Cells: Early Research
- Posted 04.19.05
- NOVA scienceNOW
(This video is no longer available for streaming.) In this 2005 segment, NOVA scienceNOW explores the promise and potential of the emerging field of stem cell research, as well as the maelstrom of emotion and politics that engulfs it. In February 2004, South Korean scientists create a worldwide media sensation by announcing that they have successfully isolated stem cells from a cloned human embryo. NOVA goes inside the Harvard labs of Doug Melton and his colleagues, who are trying to replicate the South Korean experiment.
Medical researchers see stem cells as a new way of investigating diseases such as diabetes and Parkinson's—and of treating them. But from the start, the field is torn by ethical controversy. In a $3 billion bond initiative in California, the state pits itself against the efforts of conservative politicians in Washington and elsewhere who want to shut the research down. Already ineligible for federal funding, the new science is even threatened with criminalization. The House of Representatives twice passes legislation to punish any cloning researcher with 10 years in prison and a million-dollar fine.
Transcript
STEM CELLS: EARLY RESEARCH
PBS Airdate: April 19, 2005
ROBERT KRULWICH: Nanoshells are little. They're very little. But little things matter. Remember how small a life is at the very beginning? A fertilized human egg, a few days after conception, is so small that if we go back to our pin, I could place the embryo—here we go—on the tip, on the pointy tip of a pin, which raises the question, "When life is this small, this preliminary, how do we honor what's here?"
There are scientists at Harvard—and you're going to meet them— who say it is sometimes right to use embryonic cells to investigate diseases and explore cures, while others say, "Yeah, but remember there is potential life. And we can't end that potential; that would be wrong." And we're going to show you, very precisely, what it is the scientists do to embryos, so you can see for yourself, because in the end, finding a balance between hope for cures and respect for life, that's the challenge. Here's our report from Correspondent Patty Kim.
PATTY KIM (Correspondent): Lauren Stanford is a busy 13-year-old.
LAUREN STANFORD: These ones are for, like, swim team. We won the soccer championships. That one I think I got for high honors.
PATTY KIM: And she's got another major project, keeping herself alive.
LAUREN STANFORD: At 7:15, I check my blood. At 9:30, I check my blood. At 12:11, I check my blood. At 3, or 2:45, I check my blood. At 6:00, I check my blood.
PATTY KIM: Lauren has Type 1 or juvenile diabetes. She's had it since she was in kindergarten. Unlike some kinds of diabetes, Type 1 requires constant vigilance, counting carbs, checking blood sugar and adjusting her insulin pump.
What would you say is the toughest thing about having this disease in your family?
MOIRA McCARTHY STANFORD: It's just invaded every corner of every member of our family. And everything that we're doing and she's doing is simply just keeping her alive.
PATTY KIM: Oh, wow. So how does this work? What, what does this do for you?
LAUREN STANFORD: It gives me insulin. It's giving me insulin all around...like, every 10 seconds I get insulin.
PATTY KIM: Even with all her high-tech gadgets, Lauren cannot control her blood sugar as well as a working pancreas.
There's a needle that actually goes into your arm?
LAUREN STANFORD: Yeah, but then you take it out and it's...
PATTY KIM: After 15 or 20 years with diabetes, the danger grows, possibly leading to kidney failure, heart disease, blindness.
LAUREN STANFORD: That's a glucagon. You need it when...if you have, like, a seizure. I don't want to lose my eyesight. I don't want to have brain damage. I don't want to die of early age. Like, who does? Like, that's the only thing that scares me.
MOIRA McCARTHY STANFORD: As soon as you change, you have to check your blood. Now.
PATTY KIM: But many scientists say that hope may be on the horizon.
DOUG MELTON (Harvard Stem Cell Institute): I do think we can cure it, and...our research has convinced me that that is going to be possible. Everything we learn says that it is possible.
PATTY KIM: Doug Melton has spent the last seven years searching for a cure for diabetes. His work is personal; both his children suffer from the disease.
Today, he's optimistic about their future, thanks to a hot new field in medical research which we've all heard about, embryonic stem cells. These are ells that have the potential to become nerve cells, heart cells, blood cells, any kind of cell in the human body.
Melton, now co-director of the new Harvard Stem Cell Institute, wants to turn them into insulin-producing cells so they can help diabetics.
So far, embryonic stem cells have been made mostly from frozen embryos left over from fertility treatment, and that, on its own, is controversial. But now, there's a whole new way for making them that's drawing even more fire. It has to do with cloning.
In February 2004, scientists in South Korea announced they had cloned human embryos and used them to create stem cells for research.
GEORGE DALEY: I said, "Wow, they've done it! I wish we had done it."
PATTY KIM: Dr. George Daley is a pediatrician who also studies stem cells.
GEORGE DALEY: The fact that that barrier has now been passed, I think, really motivates a lot of other scientists to say, "We can do it, too."
PATTY KIM: Now, when most of us hear the word cloning we think of the cloning of people, the stuff of science fiction films, both scary.
ACTOR (clip from Star Wars): They are totally obedient, taking any order without question.
MIKE MYERS (as Austin Powers in clip from Austin Powers: The Spy Who Shagged Me): Dr. Evil...
PATTY KIM: ...and absurd.
MIKE MYERS (as Austin Powers in clip from Austin Powers: The Spy Who Shagged Me): He is exactly like you in every way.
MIKE MYERS (as Dr. Evil in clip from Austin Powers: The Spy Who Shagged Me): Call him Mini-Me.
DOUG MELTON: I don't know any credible scientist who wants to do that, who's working on that. And I think that we, as a nation and as a world community should outlaw that. There really is no reason to be doing that.
GEORGE DALEY: I think it's important that it's clear that we're not cloning organisms, that we're cloning cells, that we're trying to create cells, not children.
PATTY KIM: So if they're not making copies of people, what kind of cloning are the scientists talking about?
Well, they say it would work like this:
Start with an egg from a woman's ovary. Remove its genes or DNA.
Then, take a cell, like a skin cell, from a patient and put its DNA into the egg. This is called nuclear transfer.
Add a few chemicals and the egg starts to divide just like a fertilized egg.
After a few days, it becomes a blastocyst, a ball of about 50 to 200 cells.
If you wanted to clone a person, then you'd have to place the blastocyst in a woman's uterus. A similar process was used to create the famous sheep, Dolly. But these scientists don't want that, so instead, and this is the step that causes so much controversy, they would break down the outer layer of the blastocyst.
It's at this point there's no turning back. The blastocyst cannot develop into a child, but the remaining cells can become embryonic stem cells, ones with the exact same genetic makeup as the patient.
The potential benefits are obvious to doctors like Leonard Zon who sees a lot of patients needing bone marrow transplants. For two thirds of them, there's no donor with an acceptable genetic match.
LEONARD ZON (Children's Hospital Boston): How are you feeling?
PATTY KIM: Even if a donor is found, the match is never perfect. Zon says, with cloning, scientists could, in theory, grow cells customized to an individual patient.
LEONARD ZON: If you used embryonic stem cell technology, you might be able to generate embryonic stem cells that have the same immune system as the patient. You'd have less chance of rejection, and we would hope that there'd be a less death rate associated with that.
PATTY KIM: With cloning, this little boy, in theory, could get bone marrow cells that perfectly match his body.
But there's much more to cloning than custom-made transplants. The thing that has scientists all over the world really excited is that cloning could allow them to do something completely new and different: make sick cells. Now why would they want to do that?
Well, let's say you could take a cell from a patient—someone like Lauren—create a clone, let it grow in the lab until it's a blastocyst, make stem cells. Then you could watch the cells as they get sick.
That's exactly what Doug Melton wants to do.
DOUG MELTON: Just like Abbott and Costello said, "Who's on first?" When you're watching these cells develop, one of them is going to make a mistake first. One of the genes in the disease cell line is going to screw up. And we want to be watching it every minute to say, "Who's on first? Who screwed up first?" If we follow those cells in a culture dish, we can get at the root cause of the disease.
PATTY KIM: If you could learn how Lauren's diabetes got started, you'd have a much better chance of curing it. This might work with lots of diseases that right now develop invisibly inside patients. Not just diabetes patients but Parkinson's patients, Alzheimer's patients.
That's why, instead of just studying healthy stem cells, scientists like Harvard's Kevin Eggan want to use cloning to create stem cells that are genetically predisposed to a disease.
KEVIN EGGAN (Harvard Stem Cell Institute): For certain diseases, it's true that cloning is the only way to do it. By taking cells from an individual which already has that disease, it does allow us to make embryonic stem cells that we know carry all the genes that are required for that disease.
GEORGE DALEY: This is one of the frontiers. It's enormously exciting research. It's very valuable. It's a whole fresh approach.
PATTY KIM: Nobody can guarantee that this approach will lead to cures, and even if it does, tangible results could be five, 10, 20 years down the road. But right now, it's unclear whether these scientists will even get started.
FATHER: This is the worst kind of science imaginable.
PATTY KIM: The Catholic Church, along with many pro-life groups, has been a major opponent of cloning.
FATHER TAD: Making human life simply to destroy it leads us right down directly on the road to barbarism.
PATTY KIM: The U.S. House of Representatives has voted twice to make all human cloning a crime. If the bill becomes law, anyone who defies the ban could go to jail for up to 10 years and face a million dollar fine.
The measure is stalled in the Senate, but Senator Sam Brownback is trying to change that.
SAM BROWNBACK: The president is ready to sign a human cloning ban. We've been blocked in the Senate. With the last election, we're going to revive efforts to try to ban human cloning.
PATTY KIM: President Bush supports some embryonic stem cell research but only on a limited number of cell lines. He opposes cloning.
GEORGE W. BUSH (President, United States of America): We should not, as a society, grow life to destroy it, and that's exactly what's taking place.
KEVIN EGGAN: I personally believe that the nuclear transfer embryos that we create are, in a sense, not new life. I would argue that that embryo that's growing in a dish, just like many other in vitro fertilization embryos, doesn't actually have the potential to go on to become an individual unless it's transferred into the uterus of a woman who's willing to carry it to term.
SAM BROWNBACK: When does that human life's significance begin? We know biologically it begins at conception. That's when your life began, that's when my life began. If I kill you as an embryo you're not here today, if you kill me as an embryo and research on me, I'm not here. So we know that, biologically.
PATTY KIM: When does a human become a human with all the legal rights and protections of a person? Much of the debate comes down to this: a human blastocyst, smaller than a grain of sand.
Whether cloned or made from the union of egg and sperm, is it the same as a person? What are its rights?
SAM BROWNBACK: What is a human embryo? What is a human clone? Is it a person or is it a piece of property? And most Americans look at this and they say, "Life beings at conception." And if that's so, that, that life...there's a sacredness to it, and we shouldn't be violating it.
DOUG MELTON: Often times this issue is couched in terms of, "When does life begin?" I think of it more as an issue, "When does a person begin?" And personhood for me is a process. The fertilized egg has the potential to become a person, but it won't necessarily become a person. Imagine you and I are sitting in an IVF clinic with my son and the fire alarm goes off. So now I have the choice of taking my son out of the room or grabbing a freezer with 100 fertilized eggs, which would I choose? I think for me that emphasizes the difference between a real life, a person who exists, and a potential.
PATTY KIM: Both the blastocyst and the child are alive. Some scientists say we're approaching a crossroads where we, as a society, have to choose. What life do we value more?
Lauren's mom, Moira McCarthy Stanford, is a Catholic and the parent of a diabetic child. Hopeful that the work might lead to a cure for diabetes, she supports cloning for research.
MOIRA McCARTHY STANFORD: To me, that ball of cells is the miracle of possibility. It's the possibility of becoming a human being if it ends up implanted in a woman. It's the possibility of becoming cells to be put in my daughter because she needs to be cured of diabetes. It's the possibility of becoming the nerve endings for a spinal cord so someone can walk again. It hasn't made its mind up yet of what to be, and, therefore, it is the possibility of all different kinds of life, whether it's new life or saving life.
ROBERT KRULWICH: That's Correspondent Patty Kim with additional reporting by Deborah Amos.
Credits
Stem Cells: Early Research
- Edited by
- Harlan Reiniger & Michael Sheehan
- Produced by
- Julia Cort, Gregory Henry, & Kyla Dunn
- Written by
- Julia Cort
NOVA scienceNOW
- Executive Producer
- Samuel Fine
- Executive Editor
- Robert Krulwich
- Senior Series Producer
- Vincent Liota
- Supervising Producer
- Andrea Cross
- Development Producer
- Kyla Dunn
- Associate Producer
- Win Rosenfeld
- Program Editor
- David Small
- Unit Manager
- Candace White
- Researcher
- Jason Spingarn-Koff
- Production Secretary
- Hong Jung
- Music
- Rob Morsberger
- NOVA scienceNOW Series Animation
- Edgeworx
- Associate Producers
- Anthony Manupelli
Mary Robertson
Cass Sapir
Ben Sweeney - Camera
- Nate Clapp
Brian Dowley
Allen Facemire
Ken Fuhr
Bob Hanna
Jack Rayzor
Jon Tichota
Joe Vitagliano
Brett Wiley
Ken Willanger - Sound Recordists
- Bob Bryan
Walter James
Anthony Rowland
Jayme Roy
George Schafnacker
Jimmy Williams
Andrew Yuncza - Audio Mix
- John Jenkins
- Animation
- Pie Design
- Additional Production by
- Simon Nasht
Annamaria Talas - Additional Music
- Mark De Gli Antoni
- Production Assistant
- Robbie Gemmel
- Compositing
- Yunsik Noh
- Special Thanks
- American Museum of Natural History
Jade Boyd
Florida State University
R. Thomas Hammond
Ann Kiessling
David Scadden - Special thanks for its decade of support for NOVA to the
- Park Foundation
- Howard Hughes Medical Institute Investigators
- Doug Melton
Leonard Zon - Archival Material
- ABCNEWS VideoSource
Rainer Albiez
Avalanche, Inc.
BBC Motion Gallery
Dr. Tony Brain & David Parker / Photo Researchers, Inc
Corbis Corporation
The Field Museum of Chicago
Gillian Darling
Susan A. Levine Photographic Art
Lubbock Metro
National Geographic Film Library
Dr. Yorgus Nikas/Photo Researchers Inc.
Bill Nipper
Paprikaas Animation Studios - Les Films D'ici, France
Popular Science
Small Times Media
Ultimate Ungulate Images - NOVA Series Graphics
- yU + co.
- NOVA Theme MUSIC
- Walter
Werzowa
John Luker
Musikvergnuegen, Inc. - Additional NOVA Theme Music
- Ray Loring
- Post Production Online Editor
- Spencer Gentry
- Closed Captioning
- The Caption Center
- NOVA Administrator
- Dara Bourne
- Publicity
- Jonathan
Renes
Diane Buxton
Olivia Wong - Senior Researcher
- Barbara Moran
- Production Coordinator
- Linda Callahan
- Unit Manager
- Lola Norman-Salako
- Legal Counsel
- Susan Rosen Shishko
- Post Production Assistant
- Patrick Carey
- Associate Producer, Post Production
- Nathan Gunner
- Post Production Supervisor
- Regina O'Toole
- Post Production Editor
- Rebecca Nieto
- Post Production Manager
- Maureen Barden Lynch
- Supervising Producer
- Stephen Sweigart
- Producer, Special Projects
- Susanne Simpson
- Coordinating Producer
- Laurie Cahalane
- Senior Science Editor
- Evan Hadingham
- Senior Series Producer
- Melanie Wallace
- Managing Director
- Alan Ritsko
- Senior Executive Producer
- Paula S. Apsell
NOVA scienceNOW is a trademark of the WGBH Educational Foundation
This material is based upon work supported by the National Science Foundation under Grant No. 0229297. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
© 2005 WGBH Educational Foundation
All rights reserved
- Image credit: (stem cell vials) © Corbis Images
Related Links
-
Stem Cells Breakthrough
Three separate teams overcome a biomedical hurdle: creating stem cells without the use of human embryos.
-

Stem Cells Breakthrough: Q&A
George Daley of Children's Hospital Boston answers questions about stem cell research.
-
Stem Cells: 2006 Update
A fraudulent South Korean researcher is unmasked, and scientists find a new technique for easing ethical concerns.
-
Stem Cells Poll
Should we create human embryonic stem cells through cloning?
-
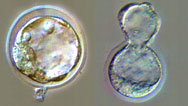
The Cloning Process
Microphotographs show step-by-step how scientists create a cloned mouse embryo to generate embryonic stem cells.
-
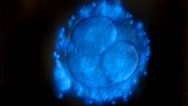
An Alternative To Cloning
Unfertilized egg cells that spontaneously develop into blastocysts offer an approach to stem cell research.