 |
 |
Frequently Asked Questions
Help/Resources | Treatments
(provided by Susan Connors, B.S., Surgical Research Laboratory, Children's Hospital,
Boston, Massachusetts)
- What is angiogenesis?
- What is an angiogenesis inhibitor?
- Are angiogenesis inhibitors being used to treat cancer? Are angiogenesis inhibitors being used alone or in combination with other therapy?
- What is a clinical trial?
- Who is eligible for a clinical trial for cancer?
- Do some patients who enroll in cancer trials receive a placebo instead of the drug?
- How can I find out about cancer trials available to me? How can I find out about clinical trials testing angiogenesis inhibitors?
- Can I use an angiogenesis inhibitor with the chemotherapy that I am on right now?
- Are these drugs available for compassionate use?
- Are angiogenesis inhibitors in clinical trial for children with cancer?
- Can angiogenesis inhibitors be used for other diseases?
- How can I find out about clinical trials for endostatin?
- When will Phase II endostatin trials begin?
- How can I find out about clinical trials for angiostatin?
- Does Dr. Folkman see patients?
| 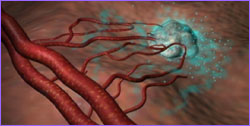 Animation of angiogenesis, with tumor
calling forth new offshoots from an existing blood vessel.
Animation of angiogenesis, with tumor
calling forth new offshoots from an existing blood vessel.
|
Q1. What is angiogenesis?
A: Angiogenesis is the process by which the body forms new, small blood vessels
called capillaries. Normally this process is short-lived and beneficial, as in
wound healing, reproduction, and fetal development. Under some abnormal
conditions, angiogenesis can develop and be detrimental, as it contributes to
the growth of cancer and other diseases. This abnormal angiogenesis allows
tumors to grow and metastasize (spread to other organs or other parts of the
body) by providing nutrients and growth factors brought in by the new blood
vessels. Virtually all tumors are dependent on angiogenesis for their
growth.
Q2. What is an angiogenesis inhibitor?
A: An angiogenesis inhibitor is a naturally occurring or synthetic molecule that
can interfere with or "block" angiogenesis. Since abnormal angiogenesis rarely
turns itself off spontaneously, the goal of antiangiogenic therapy is to stop
the formation of new blood vessels and to break up the existing abnormal blood
vessels. Angiogenesis inhibitors target new, growing blood vessels and do not
affect normal resting blood vessels in the body.
Q3. Are angiogenesis inhibitors being used to treat cancer? Are angiogenesis
inhibitors being used alone or in combination with other therapy?
A: Angiogenesis inhibitors are a new class of drug being studied in clinical
trials in patients with advanced cancer that has failed to respond to
conventional therapy. These inhibitors are not yet approved by the Food and
Drug Administration (FDA) for use outside of clinical trials. Many angiogenesis
inhibitors are under development and investigation, and more than 20 different
angiogenesis inhibitors are in clinical trial for cancer at medical centers in
the United States.
 Eventually doctors may add
antiangiogenesis drugs to more traditional cancer-fighting approaches, such as
chemotherapy.
Eventually doctors may add
antiangiogenesis drugs to more traditional cancer-fighting approaches, such as
chemotherapy.
|
|
Angiogenesis inhibitors target the blood supply feeding the tumor, while
surgery, standard chemotherapy, and radiation therapy target the tumor
itself. Eventually, angiogenesis inhibitors may be added to chemotherapy
or to radiation therapy or to other treatments such as vaccine therapy and gene
therapy. Also, in the future, combinations of angiogenesis inhibitors may be
used together.
|  All cancer drugs, whether for
conventional chemotherapy or unconventional angiogenesis-inhibiting treatment,
must go through clinical trials.
All cancer drugs, whether for
conventional chemotherapy or unconventional angiogenesis-inhibiting treatment,
must go through clinical trials.
|
Q4. What is a clinical trial?
A: A clinical trial is a strictly monitored scientific study, in which new
treatments are tested on patients in a hospital or clinic. Clinical trials
determine whether a new treatment is safe, effective, and a better alternative
to current standard therapy. All clinical trials involving humans must
be approved by the institution's review board. This review board is made up of
physicians, administrators, scientists, ethicists, and laymen. All patients
participating in a clinical trial will go through an informed consent process
before starting treatment. This process will include a discussion between
doctor and patient about all aspects of the investigational therapy, including
possible risks and benefits of treatment. A patient can withdraw from a
clinical trial at any point, for any reason, even after consent is given and a
consent form is signed. Treatment found to be safe and effective can be made
available for widespread use, after approval by the FDA. New investigational
drugs are usually for patients whose disease has not responded to current
standard therapy and should not be used as substitutes for proven effective
treatment.
Clinical trials take place in three different phases:
Phase I
After extensive testing on animals in the laboratory, a small number of
patients (usually fewer than 100) are enrolled in a Phase I clinical trial.
This initial phase of testing will determine safety, common side effects,
information about dosage, and the best way to give the drug. At this stage,
little is known about how effective the new treatment will be.
Phase II
Phase II clinical trials continue to evaluate the safety of the treatment and
provide some information about the effectiveness of the drug. Phase II trials
include a larger number of patients and may focus on a particular type of
cancer.
Phase III
Phase III clinical trials are conducted to confirm the effectiveness of the
drug. These studies often compare the experimental treatment with current
standard treatment or combine the experimental treatment with standard
treatment and compare this to standard treatment alone. These trials usually
consist of two groups of patients: the "treatment group," which is given the
experimental treatment, and the "control group," which is given the standard
treatment. Phase III trials usually enroll over 1,000 patients.
Q5. Who is eligible for a clinical trial for cancer?
A: Most cancer centers have a number of clinical trials available for patient
participation. These clinical trials follow a written protocol, which is a set
of rules for treatment. This protocol lists diagnostic tests, procedures,
medications, doses, length of treatment, inclusion criteria, and exclusion
criteria. A patient's eligibility may depend on factors such as current medical
condition, extent of disease, type of tumor, and prior therapy. A member of the
study research team will review eligibility criteria with each patient before
enrollment. Most clinical trials require that a patient's disease be refractory
(unresponsive) to conventional therapy, or be a disease for which there is no
effective known curative therapy.
Q6. Do some patients who enroll in cancer trials receive a placebo instead of
the drug?
A: Cancer trials rarely use placebos (a dummy treatment containing no drug). In
trials that compare the experimental treatment to the best standard treatment,
patients are usually "randomized" (chosen at random) to one of two different
groups: the "treatment group," which is given the experimental treatment, and
the "control group," which is given the standard treatment. In this situation,
the patient gets assigned to one group or the other and may or may not receive
the experimental therapy.
Q7. How can I find out about cancer trials available to me? How can I find out
about clinical trials testing angiogenesis inhibitors?
A: There are many hundreds of cancer trials being conducted in the United States
and abroad. Most of the trials are sponsored by government agencies and
pharmaceutical companies, and are conducted at cancer centers, hospitals, and
some doctor's offices. There is no single resource that lists all cancer
trials. You can ask your oncologist about clinical trials being conducted at
the center where you are being treated. In addition, many cancer centers and
pharmaceutical companies have Web sites listing available clinical
trials.
 Hundreds of cancer trials for all kinds of
treatments are taking place worldwide. For details, see the National Cancer
Institute Web site.
Hundreds of cancer trials for all kinds of
treatments are taking place worldwide. For details, see the National Cancer
Institute Web site.
|
|
The National Cancer Institute (NCI) has a comprehensive database of information
about cancer research studies. This database includes information about
specific types of cancer, information about finding ongoing trials (including a
list of angiogenesis inhibitors in clinical trial), and a list of
NCI-designated Cancer Centers by geographic region. Specific trials are listed
in a database produced by the National Cancer Institute called PDQ (physician
data query). In addition to specific trials, the PDQ describes the latest
advances in cancer screening, prevention, and treatment. This information is
available to patients and their families as well as to health-care
professionals. For more information about these clinical trials, you may
contact the National Cancer Institute's Cancer Information Service at
1-800-4-CANCER (1-800-422-6237) or on the Internet at
http://www.cancer.gov/cancer_information/ or http://cancertrials.nci.nih.gov.
Before you make a decision about enrolling in a clinical trial, including a
trial testing an angiogenesis inhibitor, you may want to confer with your
physician. He or she can help you put information about clinical trials into
perspective and help you decide what is appropriate for you.
|  Judah
Folkman can only wait and watch as his team's angiogenesis inhibitors go
through clinical trials.
Judah
Folkman can only wait and watch as his team's angiogenesis inhibitors go
through clinical trials.
|
How long will I be treated with an angiogenesis inhibitor?
Since angiogenesis inhibitors are in the early stages of testing, it is not
known how long this type of drug will have to be used to be most effective.
Information about a specific drug's effectiveness, toxicity, long-term side
effects, and the development of drug resistance or lack of, will help determine
how long the course of treatment with an angiogenesis inhibitor will be. When
you are part of a clinical trial testing an angiogeneis inhibitor, you will be
expected to participate for a pre-defined minimum period of time, depending on
the phase of the trial. However, in most clinical trials, if a patient is not
experiencing any unexpected toxicities, and the tumor is stable or shrinking,
the patient may continue on treatment.
Q8. Can I use an angiogenesis inhibitor with the chemotherapy that I am on right
now?
A: Angiogenesis inhibitors currently in clinical trial are part of protocols
written specifically to test these drugs for safety and effectiveness. Until
these drugs are approved by the FDA, they must be tested in strictly monitored
studies. Only anticancer drugs that are part of the investigational protocol
may be used.
Q9. Are these drugs available for compassionate use?
A: Permission for compassionate use of a drug is given by the FDA and drug study
sponsor. Until more is known about a drug's safety and effectiveness, it is not
available for individual patients who are not part of a clinical trial.
Q10. Are angiogenesis inhibitors in clinical trial for children with
cancer?
A: Angiogenesis inhibitors must be tested for safety in adult cancer patients
first. If a drug proves to be safe, the FDA may allow it to be tested in a
clinical trial for children with cancer. At this time there are only a few
angiogenesis inhibitors in clinical trial for children and these studies are
limited in number and size. For information about angiogenesis inhibitors in
clinical trial for children with cancer, you may consult your child's
oncologist or contact the National Cancer Institute's Cancer Information
Service at 1-800-4-CANCER (1-800-422-6237) or on the internet at
http://cancernet.nci.nih.gov or http://cancertrials.nci.nih.gov.
 Robert D'Amato, a
member of Judah Folkman's team, discovered that thalidomide was a potent
angiogenesis inhibitor.
Robert D'Amato, a
member of Judah Folkman's team, discovered that thalidomide was a potent
angiogenesis inhibitor.
|
|
Q11. Can angiogenesis inhibitors be used for other diseases?
A: The role of angiogenesis and its contribution to the development of other
diseases is being widely studied. In the future, these diseases may be treated
with angiogenesis inhibitors.
Currently, angiogenesis inhibitors are in clinical trial for certain eye
diseases. For information about these clinical trials for eye disease, you may
consult your physician or contact the Macular Degeneration Foundation at
1-888-633-3937 or www.eyesight.org.
Q12. How can I find out about clinical trials for endostatin?
A: EntreMed is conducting several Phase I safety studies of endostatin. These
trials are nearing completion and limited openings are available. Enrollment in
these trials is restricted to patients who live in the same area as the trial
center. Patients seeking additional information about these trials should call
the University of Wisconsin (Madison) Cancer Connect Line at 1-800-622-8922 or
608-262-5223, or MD Anderson Cancer Center (Houston, Texas) Information line at
1-800-392-1611 (Select Option 3) or 713-792-6161.
Q13. When will Phase II endostatin trials begin?
A: Plans for Phase II clinical trials for endostatin are underway. These trials
will help determine endostatin's effectiveness. Data from the Phase I trials
(for example, results on routes of administration, schedules of administration,
and dosages) will be used to design the Phase II trials. These Phase II trials
are expected to begin during the second quarter of this year. Phase II clinical
trial sites have not yet been selected, and there are no "waiting lists" for
patients at this time.
Q14. How can I find out about clinical trials for angiostatin?
A: In the United States, there are two Phase I clinical trials evaluating the
safety of angiostatin in humans. These trials are sponsored by EntreMed, Inc.
and are being conducted at Thomas Jefferson University Hospital in
Philadelphia, Pennsylvania. One of the studies is testing angiostatin as a
single agent, and the other study is testing angiostatin in combination with
radiation therapy. Enrollment in these trials is limited to patients who live
in the same area as the trial center. Patients seeking additional information
about these trials may call 1-800-JEFF-NOW or contact the Thomas Jefferson
University Web site at www.kcc.tju.edu.
|  Dr. Folkman
does research full-time and no longer sees patients.
Dr. Folkman
does research full-time and no longer sees patients.
|
Q15. Does Dr. Folkman see patients?
A: Dr. Folkman is a physician, Professor of Surgery and Cell Biology at Harvard
Medical School, and Director of the Surgical Research Laboratory at Children's
Hospital in Boston. He is devoting his full effort to scientific research and
does not see patients.
There are many excellent oncologists who are treating patients throughout the
United States and worldwide, and most cancer centers have a referral service
for patients seeking an oncologist. For a list of NCI-designated cancer centers
by geographic region, you may contact the National Cancer Institute's Cancer
Information Service at 1-800-4-CANCER (1-800-422-6237) or on the Internet at
http://cancernet.nci.nih.gov.
Dr. Folkman Speaks |
Cancer Caught on Video
Designing Clinical Trials |
Accidental Discoveries |
How Cancer Grows
Help/Resources |
Transcript |
Site Map |
Cancer Warrior Home
Editor's Picks |
Previous Sites |
Join Us/E-mail |
TV/Web Schedule
About NOVA |
Teachers |
Site Map |
Shop |
Jobs |
Search |
To print
PBS Online |
NOVA Online |
WGBH
© | Updated February 2001
|
|
|